The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
In a recent publication, Gabriela Haynes (2022) argued that Archaeopteryx should be considered a bird rather than a dinosaur, that birds and dinosaurs are obviously distinct, that statistical baraminology is too influenced by evolutionary thinking to be of use to creationists, and that scientists should only use Linnaean taxonomy in classifying organisms. We are glad to see other creationist scientists engaging with the beauty and complexity of God’s design, especially in the area of paleontology and the complex issues surrounding dinosaurs, birds, and feathers. Nonetheless, we recognize multiple issues with Dr. Haynes’ assumptions, methodologies, and conclusions: 1) there appears to be an unfamiliarity with cladistics and statistical baraminology, and specifically how these disciplines build and employ morphological datasets; 2) discussions of dinosaurs, birds, and feathers are, at times, imprecise and inconsistent with the current state of vertebrate anatomy and taxonomy; and 3) several biblical and philosophical conclusions extend beyond the claims of Scripture. Based on abundant evidence from numerous taxa, we recognize that feathers (including bristles, down, and pennaceous types) are found broadly across coelurosaurian theropods, that the anatomy of Archaeopteryx is strikingly similar to dromaeosaurid and troodontid theropod dinosaurs, and that the morphological datasets which tabulate the physical character states of these taxa are both accurate and robust. Employing these datasets through baraminological methods has allowed creation researchers to distinguish various kinds of dinosaurs and birds. Importantly, the presence of feathers on dinosaurs neither implies nor entails an evolutionary connection between these groups.
Introduction
Among living animals, birds are incredibly unique. There is only one other group of extant flying vertebrates (bats), and no other living animal group possesses the complex, branching integumentary structures that we call feathers. The discovery of Archaeopteryx in the mid-nineteenth century was a surprise to scientists, a creature with the feathers of a bird yet possessing other features not recognized among modern birds, including true teeth, a long bony tail, and clawed manual digits (now known to be relatively common in birds, occurring in at least 170 genera in 22 orders [Wein and Schwing 2017]). Richard Owen (1863) thought Archaeopteryx was an unusual bird, whereas other researchers concluded it was a reptile with skin structures that merely resembled feathers (for example, Wagner 1862). The debate as to what sort of creature Archaeopteryx was continues up to the present day, especially since many scientists have posited that it is an example of a transitional form linking dinosaurs and birds (for example, Foth, Tischlinger, and Rauhut 2014; Huxley 1870; Ostrom 1976). It would be more than a century from Archaeopteryx’s discovery for fossils of animals traditionally assigned to dinosaurs, yet sporting feathers, to be unearthed. This includes representatives of the Dromaeosauridae, Troodontidae, Ornithomimosauria, and Oviraptoridae, each of which now include taxa possessing pennaceous feathers (for example, Qiang et al. 1998; van der Reest, Wolfe, and Curry 2016; Xu et al. 2003a; 2003b; 2017, respectively). Still other dinosaur fossils display evidence of filamentous structures that may or may not be homologous with feathers (for example, Sinosauropteryx, Psittacosaurus, Tianyulong, and Kulindadromeus).
These discoveries have started a lively discussion in the creationist literature concerning the nature of these creatures and what they mean for a biblical worldview. Two main perspectives have been put forth. The first holds that these fossils are examples of properly-identified dinosaurs that possess a range of feather morphologies (for example, Garner, Wood, and Ross 2013; McLain, Petrone, and Speights 2018; Surtees 2021). The second holds that true feathers have only been recovered on birds, resulting in proposals to reassign some feathered dinosaurs to birds and/or argue that degraded collagen has been mistaken for feathers among some dinosaurs (for example, Cserhati, Thomas, and Tay 2020; Haynes 2022; Thomas and Sarfati 2018).
A recent article by Haynes critiqued previous creationist research arguing for feathered dinosaurs and claimed that “there is no reason for Archaeopteryx to be anything other than a bird” (Haynes 2022, 297). We find that this conclusion faces substantial challenges. In this response we address several relevant issues and provide alternatives that are consistent with both the biblical account and the fossil record. These include 1) aspects of classification and analytical taxonomy; 2) anatomical features of dinosaurs, birds, and feathers; 3) the anatomy of Archaeopteryx; and 4) careful attention to the claims of Scripture.
Classification and Taxonomy
Haynes argues that the “presence of feathers has been the key to classifying an animal as a bird based on the classical, conventional, and traditional taxonomy developed by Linnaeus in the eighteenth century” (Haynes 2022, 288). Later she states, “following the classical, traditional Linnaean classification and reasoning, it is concluded that there is no reason for Archaeopteryx to be anything other than a bird” (Haynes 2022, 297). However, Linnaeus’ entry for Aves in the groundbreaking tenth edition of Systema Naturae noted that a defining feature of birds is that they are edentulate (lacking teeth; Linné 1806, iv). Based on this, Archaeopteryx and numerous other fossil avians cannot be birds because they possess teeth (for example, Bohaiornis, Ichthyornis, Hesperornis). For Haynes to argue that Archaeopteryx is a bird requires that she, too, must modify or reject Linnaeus’ definition, a practice that she heavily criticizes.
We submit that it is entirely reasonable and necessary to change and update the definitions and contents of taxonomic groups as we learn more about nature. Indeed, Linnaeus himself constantly updated Systema Naturae over its 13 editions (the last published posthumously), and some of these changes were quite dramatic at the time. For example, when Linnaeus introduced the binomial nomenclature of genus and species in the tenth edition, he also (among many other changes) reclassified whales as mammals rather than fish. It is surprising, then, that Haynes disparages those who change the definitions for taxonomic groups throughout her article while simultaneously acknowledging that “in all scientific fields and endeavors, scientists do not understand everything, which is why there is a need to keep researching . . . scientists also have fallible and finite minds in this fallen world as they try to understand the creatures created by the Creator’s perfect, infinite, and creative mind” (Haynes 2022, 287–288). We agree with these points, and it is precisely because our knowledge is incomplete, requires frequent updates, and is prone to errors and corrections, that adjustments and updates to biological definitions are necessary and welcome features of good scientific work.
Distinguishing Descriptive Anatomy from Taxonomy
The primary thrust of Haynes’ article is that creationists who have accepted feathered dinosaurs have succumbed to evolutionary thinking. She cites as evidence the use of cladistic datasets and terminology by creationists doing statistical baraminology, rather than traditional Linnaean systematics. “Cladistics,” she writes, “has faulty and unbiblical premises that do not fit within a young-earth creation perspective . . . since the assumptions of cladistics (evolution and common ancestry) are not rooted in the Scriptures, it does not seem reasonable to borrow this method to explain any fossil data within the young-earth creationist framework” (Haynes 2022, 292). Haynes sees cladistics as anti-biblical and Linnaean taxonomy as biblical, because the former is based in evolution/common ancestry while the latter is based upon concepts of created kinds.
However, this is an overly simplistic view. Linnaeus’ taxonomic method was certainly intended to reflect Genesis’ account of God creating according to kinds, but it was also steeped in a Platonic conception of ideal forms. For most of his life, Linnaeus believed that the species he described were in fact the created kinds (species is the Latin word for “kind”), and that these species were fixed and immutable. His views on species fixity changed as he became aware of evidence for hybridization in both plants and animals (Garner 2009). So while Linnaeus’ concepts were biblically minded, they were also strongly influenced by non-biblical philosophy. Furthermore, Linnaeus’ taxonomic method was later co-opted by evolutionary workers who argued that the nested hierarchies in which species were classified were best explained by common ancestry. It is therefore quite clear that employing Linnaean systematics does not guarantee that one’s methods or results are biblical.
Haynes is correct to point out that cladistics was developed as a purely evolutionary taxonomic system that assumes universal common descent. However, it is an overreach to claim that, due to its origins in evolutionary studies, cladistics is always and invariably unbiblical. After all, cladistics is simply a method of hierarchically arranging organisms (individuals, species, genera, and so on) by plotting them on trees based on the proportions of how similar their characteristics are. When we use this method to investigate actual biological descent and relatedness as creationists, then the method should be restricted to questions of ancestry within created kinds, rather than between them. Such applications are non-controversial among young-earth creationists. For example, cladistic methods are employed by Jeanson (2022) in his approach to tracing the genetic signature of the Y-chromosome among human beings. Jeanson builds a cladistic tree of genetic ancestry going back to Noah’s three sons and fits the genetic haplotypes of extant Homo sapiens onto its branches. Thus the objection is not to cladistics per se, but to its application in generating hypotheses that posit common ancestry among separately created kinds. Like all other young-earth creationists, we reject such applications as inappropriate uses of cladistic methods (see McLain, Petrone, and Speights [2018] for further discussion).
These issues of the nature and applicability of taxonomic schemes are important and warrant further exploration, but the larger issue with Haynes’ critique is that she confuses descriptive and comparative anatomy with classification methods. Descriptive anatomy focuses on the physical features of an organism. Archaeopteryx is Archaeopteryx because of the particular attributes of its skull, limbs, vertebrae, etc. Whether Archaeopteryx is a dromaeosaurid or avialan is the question that classification must address because the terms “dromaeosaurid” and “avialan” have meanings beyond the descriptive anatomy of Archaeopteryx. These broader terms are baskets, and the taxa are the apples and oranges we are trying to sort into appropriately similar versus dissimilar groups. With this in view, cladistic analyses, baraminological studies, and Linnaean higher-rank assignments are not the description of the organism, they are the means of producing hypotheses of classification. These hypotheses are then tested when new discoveries or corrections to errors are made among the taxa in question.
Cladistics and Baraminology
While it is beyond the scope of this paper to fully articulate and defend baraminological methods (see Wood 2021a for a recent treatment), certain issues are relevant to this discussion and can be addressed. In particular, Haynes demonstrates a deep skepticism of morphological character datasets, which we have found to be a common position among creationists critical of baraminological studies (for example, DeWitt, Habermehl, and Menton 2010; Sanders and Cserhati 2022). Haynes writes,
The available published data are taken from the evolutionist literature and can be insufficient, lacking, misinterpreted, misidentified, or misrepresented . . . Furthermore, the data used in the method can be arbitrary and subjectively chosen, so the evolutionary bias in the data needs to be considered. If there are problems with the data, that will affect the method’s outcome since it is statistical . . . That is, a lack of reliable data and methodological application means a lack of reliable results. (Haynes 2022, 290, 292)
These are strong accusations, yet Haynes does not provide a single example of erroneous observations, coding errors, misrepresentations, or evolution-influenced biases in any published morphological dataset. Rather, Haynes presents the differing conclusions for the placement of Archaeopteryx within Deinonychosauria or Avialae in several evolutionary analyses as if they are self-evident arguments for bias and subjectivity in both morphological datasets and cladistic evaluations (Haynes 2022, 289). We might ask why, if the data are hopelessly biased by evolutionists, do various cladistic analyses produce different results? The inability to produce a unified consensus argues against intentional bias, not for it. To address these claims of systematic bias and error, we turn to how these datasets are constructed, how they are employed in statistical baraminology, and how baraminological and cladistic approaches differ.
Morphological character datasets used in studies of fossil organisms are composed of hundreds, even thousands, of highly detailed anatomical evaluations. Character states are numerically coded (0, 1, 2, etc.) according to the number of variations seen among the taxa being evaluated. If a character cannot be ascertained due to incomplete/missing fossil material, a “?” is entered, or if a character is irrelevant to a particular taxon, a “?” or “-” is entered, both of which are computationally distinct from codes used to identify character states (0, 1, 2, etc.). This process of describing and evaluating fossils is empirical science, since descriptive anatomy involves observable data that can be checked by other researchers. Of course, various experts may code for some different features, or may disagree whether a taxon displays a specific character state or not. Additionally, researchers use different parameters by which the cladogram will be constructed, such as parsimony, maximum-likelihood, neighbor-joining, character weighting, etc., and these differing parameters influence aspects of the resultant cladistic trees (see Simões et al. 2017 for an evaluation of multiple cladistic parameters in their study of mosasaurs). But none of these are “worldview” issues, they are minor disagreements among specialists affecting a small portion of the datasets and preferences for the mode of evaluation.
To illustrate the empirical nature of morphological datasets, Table 1 is a list of 20 characters chosen at random (using the Google Random Number Generator and then placed in ascending order) from 853 characters in the Brusatte et al. (2014) dataset. This was one of the datasets used (with slight modification by Cau, Brougham, and Naish (2015) in the statistical baraminological analysis of McLain et al. (2018). Character 80, for example, asks whether the maxilla (the main upper jaw bone) bears teeth, where “0” is yes and “1” is no. Character 610 asks about the dorsoventral (top to bottom) depth of the basioccipital (a bone at the back of the skull), and specimens are coded as either having a depth less (0) or greater (1) than the depth of the occipital condyle (the bony knob that articulates with the first vertebra). Evaluating these morphological character states requires detailed anatomical expertise and the answers are independent of whether the researcher is a creationist or an evolutionist (examples of young-earth creationists producing published morphological datasets include Sanders 2016 and Wood 2021b). There may be some questions or disagreements: for instance, paleontologists may differ on how to code character 478 for a skull that is approximately 40% of the trunk length. Nevertheless, these do not result in the systematic corruption of the fossil data.
| Character | Description | States | Falcarius | Deinonychus | Archaeopteryx | Confusciusornis |
|---|---|---|---|---|---|---|
| 5 | Postorbital, ventral ramus, orientation (unordered) | “0: parallels quadrate, lower temporal fenestra rectangular in shape 1: oriented strongly obliquely relative to quadrate, jugal and postorbital approach or contact quadratojugal to constrict lower temporal fenestra 2: oriented anteroventrally relative to the long axes of the quadrate and lacrimal, angle of postorbital ventral ramus long axis with the lacrimal long axis (if lacrimal is approximately vertical) greater than 30 degrees” | ? | 0 | 0 | 0 |
| 32 | Jugal, dorsoventral height beneath lower temporal fenestra | “0: tall, twice or more as tall dorsoventrally as it is wide transversely 1: very short, jugal rod-like” | ? | 0 | 0 | 0 |
| 77 | Lower jaw, glenoid articular surface for mandible, anteroposterior length | “0: approximately as long as distal quadrate condyles 1: twice or more as long as distal quadrate condyles, allowing anteroposterior movement of mandible” | ? | 0 | 0 | 0 |
| 80 | Maxilla, teeth | “0: present 1: absent” | 0 | 0 | 0 | 1 |
| 96 | Cervical vertebrae, shape of anterior articular surface of anterior cervical centra | “0: subcircular or square in anterior view 1: distinctly wider than high, kidney shaped” | 1 | 1 | ? | ? |
| 99 | Cervical and anterior trunk vertebrae, form (unordered) | “0: amphiplatyan or weakly opisthocoelous (anterior surface flat or weakly convex, posterior surface is flat or weakly concave) 1: strongly opisthocoelous (anterior surface is convex and posterior surface concave 2: at least partially heterocoelous” | 0 | 0 | 0 | ? |
| 225 | Pedal phalanx II-2, flexor heel, form | “0: small and asymmetrically developed only on medial side of vertical ridge subdividing proximal articulation 1: heel long and lobate, with extension of midline ridge extending onto its dorsal surface” | ? | 1 | ? | ? |
| 310 | Thoracic vertebrae, form of articular surfaces | “0: at least part of series with round or ovoid articular surfaces (e.g. amphicoelous/ opisthocoelous) that lack the dorsoventral compression seen in heterocoelous vertebrae 1: series completely heterocoelous” | 0 | 0 | 0 | 0 |
| 324 | Sternum, pneumatic foramina in the depressions (loculi costalis) between rib articulations (processi articularis sternocostalis) | “0: absent 1: present” | ? | ? | ? | 0 |
| 334 | Scapula and coracoid, form of articulation (unordered) | “0: pit-shaped scapular cotyla developed on the coracoid, and coracoidal tubercle developed on the scapula (“”ball and socket”” articulation) 1: scapular articular surface of coracoid convex 2: flat” | 2 | 2 | ? | 0 |
| 345 | Coracoid, medial surface, area of the foramen n. supracoracoideus (when developed) | “0: strongly depressed 1: flat to convex” | 0 | 0 | 0 | ? |
| 434 | Caudal vertebrae, middle to posterior caudals, anteroposterior length (ordered) | “0: shortened, less than 1.5x length of dorsal vertebrae (where known) and anteroposterior length of centrum less than twice its maximum mediolateral width 1: 1.5x-2x or less the length of dorsal vertebrae 2: 3x-4x length of dorsal vertebrae” | 0 | 1 | ? | 0 |
| 478 | Skull, anteroposterior length | “0: less than 40% trunk length 1: greater than 40% trunk length” | ? | ? | 0 | 1 |
| 557 | Squamosal, pneumaticity: posterior process, inflated by squamosal recess (ordered) | “0: absent 1: present as a deep, concave depression on the ventral surface of the main body 2: present as a deep, concave depression on the ventral surface of the main body, and extending posteriorly to inflate the squamosal posterior process” | ? | 0 | ? | ? |
| 582 | Parietal, skull table between supratemporal fossae, width | “0: braod, more than 10% of the mediolateral width of the fossa 1: extremely reduced, sagittal crest or crests (if present) pinched between opposing fossae” | ? | ? | 0 | ? |
| 610 | Basioccipital, basal tubera, dorsoventral depth | “0: less than depth of occipital condyle 1: greater than depth of occipital condyle” | 1 | ? | 0 | ? |
| 763 | Humerus, shape of internal tuberosity in anterior view (ordered) | “0: triangular or rounded, not discretely separated from remainder of humerus 1: rectangular, separated from the humeral head by a small but distinct notch 2: rectangular and hypertrophied, separated from the humeral head by a large notch” | 1 | 1 | 1 | ? |
| 784 | Dentary teeth, shape of mesial (anterior) teeth | “0: not conical (i.e., ziphodont or lanceolate) 1: conical” | 1 | 0 | 0 | ? |
| 798 | Metacarpal I, rectangular buttress on ventrolateral aspect of proximal surface that underlies ventromedial surface of metacarpal II | “0: absent 1: present” | 1 | 0 | ? | ? |
| 843 | Tibia, position of medial ridge on posterior surface of distal end (ordered) | “0: displaced laterally, positioned lateral to the medial edge of the distal tibia by approximately 25-33% of the mediolateral width of the distal tibia 1: positioned lateral to the medial edge of the distal tibia approximately 10-20% of the mediolateral width of the distal tibia 2: positioned medially, positioned at approximately the posteromedial corner of the distal tibia in distal view” | 1 | 2 | ? | ? |
The Brusatte et al. (2014) dataset has been adopted, added to, and improved upon by several other workers (for example, Cau, Brougham, and Naish 2015), which indicates that other researchers see the work as rigorous and have desired to add to the dataset rather than start over from scratch. We also recognize that these datasets are reliable, which is why they are used in statistical baraminology to evaluate fossil groups. We must resist calls to disregard valid, empirical data merely because they were collected and published by evolutionists. These sorts of statements move beyond reasonable caution into unrestrained and debilitating skepticism. After all, it is not evolution that informs us whether or not Archaeopteryx has teeth in its maxilla, the fossils adjudicate this question.
The key, then, is to use the best data available, to recognize its strengths and weaknesses, and to apply proper analytic techniques. Haynes claims “Some creation scientists have also used the assumptions of cladistics and its results to run baraminological analyses” (Haynes 2022, 289), and other creationist authors imply that statistical baraminologists uncritically take datasets at face value (for example, Sanders and Cserhati 2022). These charges are impossible to square with the 25-year history of statistical baraminology. First, baraminological studies differ sharply from cladistics, in that they 1) do not assume universal common ancestry, 2) utilize different computational methods, 3) identify both similarities and differences among taxa, and 4) are not tethered to tree-based representations of relationships. Second, readers can consult any number of robust baraminological studies, which frequently include substantial efforts to evaluate the morphological datasets that inform them (for example, Clausen and McLain 2021; Wood 2016). Moreover, it is incumbent upon critics of the use of morphological datasets, such as Sanders and Cserhati (2022) whom Haynes relies on for her critique, to provide specific and demonstrable evidence of where and how a given dataset is erroneous, corrupted, or invalid. Assertions of general evolutionary bias do not constitute a sufficient argument for dismissing vast quantities of anatomical observations en masse, and thus such assertions do not disqualify statistical baraminology as a young-earth creation endeavor.
Taxonomic identification and character determination are objective and empirical data with minimal bias. The goal in using comparative anatomy for understanding taxonomic relationships should be to get as much data from throughout the body as possible to look for the proportion of overall similarities and differences, which is exactly what happens in cladistic and statistical baraminological studies. It is the analytical techniques that differ in purpose, method, and application: cladistics is a method that assumes common ancestry among all of the taxa investigated, while baraminology asserts that organisms can be assigned to different created kinds that do not share common ancestry. Has it not been creationists who, for decades now, have been saying “we have the same data, but different interpretations”?
Dinosaurs and Birds
At any given time, taxonomic definitions are specific and based on empirical data. Yet they are also subject to revision because taxonomy is an active scientific discipline whose findings are tentative. This was true for Linnaeus. As noted above, when it became apparent that whales were air-breathing mammals he changed their taxonomic location from Pisces to Mammalia (Linné 1806). Thus, it is no surprise that taxonomic definitions for terms like “dinosaur” and “bird” have been revised frequently over time, and we therefore cannot agree with Haynes’ claim that “Cladistics terms and definitions are used in a very subjective and arbitrary way” (Haynes 2022, 292). Rather, taxonomic definitions are precise and accurate given the state of knowledge at the time.
Central to the debate over feathers and dinosaurs, then, are the definitions of “feather” and “dinosaur.” Any definition for feathers must include details about their structure and composition. Xu and Guo (2009, 321) define modern feathers as “complex integumentary appendages formed by hierarchical branches of rachis, barbs, and barbules which are composed of Φ-keratins and grow from a follicle.” Modern feathers include seven different forms, including pennaceous types (flight, tail, contour, and semiplume), filamentous down, filoplume, and bristles (figs. 1 and 2). There are even some bizarre feather types, like the keratinous quills found on the wings of cassowaries in place of their remiges (fig. 3; also see Saber and Hassanin 2014). McLain, Petrone, and Speights (2018) detailed the various types of feathers found in fossil birds and dinosaurs.
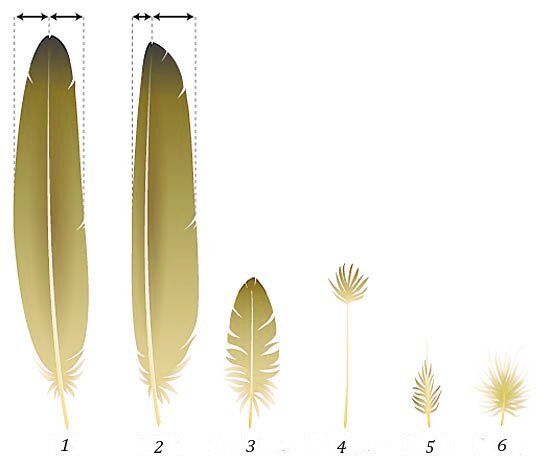
Fig. 1. Types of feathers in modern birds: 1) symmetrical tail feather; 2) asymmetrical flight feather; 3) contour feather; 4) filoplume; 5) semiplume; and 6) downy feather. Not illustrated is a bristle, which has an elongate, bare rachis (like filoplume but without the tuft at the top). Anaxibia. “Types of feathers.” https://commons.wikimedia.org/w/index.php?curid=18722698. CC BY-SA 3.0.

Fig. 2. Close-up of the face of a green barbet (Psilopogon viridus) displaying elongate, bare bristle feathers projecting around the beak and the covert feathers over the body. Similar structures are found alongside numerous dinosaur fossils. L. Shyamal. “Rictal bristles of a Small Green Barbet (Megalaima viridis).” https://commons.wikimedia.org/w/index.php?curid=3489015. CC BY-SA 2.5.

Fig. 3. Photograph of the single-wattled cassowary (Casuarius unappendiculatus) showing the long, bare quills that come off the wings. Quartl. “Single-wattled Cassowary (Casuarius unappendiculatus) in the Walsrode Bird Park, Germany.” https://commons.wikimedia.org/wiki/File:Casuarius_unappendiculatus_qtl1.jpg. CC BY-SA 3.0. Original photograph modified with an arrow pointing to the quills.
Dinosauria is an archosaurian group characterized by a variety of skeletal traits possessed by its members. These include, but are not limited to (Nesbitt 2011):
- Epipophyses present on the postaxial anterior cervical vertebrae;
- A deltopectoral crest located 30% or further down the length of the humerus;
- The radius is shorter than 80% of the humerus length;
- A perforated acetabulum;
- Fourth trochanter is a sharp flange.
Nesbitt (2011) identifies a total of 12 dinosaur-unique traits and 13 additional traits that may also qualify (Haynes 2022, 210). Beyond these, there are other traits that are characteristic of dinosaurs but are also shared with similar non-dinosaurian groups (for example, silesaurids), or are nearly, but not completely, found among all dinosaurian taxa. For example, dinosaurs typically have three or more fused sacral vertebrae, stand with their limbs directly under their bodies, and possess an astragalus that extends over the distal anterior surface of the tibia. All of the above anatomical features, plus additional ones, are the means by which paleontologists can identify whether an organism is or is not a dinosaur. The features observed that further define specific dinosaur groups, genera, and species are similarly (and increasingly) precise. In some cases, a taxon may lack some of these features but is still considered a dinosaur on the basis of possessing many of the other traits. This is not unusual in biology. For instance, all biologists consider snakes to be in Tetrapoda even though they have no legs. Rather, the myriad of reptilian traits that snakes possess supersedes their limbless condition.
In contrast to the rigorous anatomical details noted above, Haynes’ approach to definitional issues is muddled. First, Haynes claims that “bird” is defined as those organisms “having actual modern-looking feathers” (Haynes 2022, 293). What exactly is meant by this is unclear. As mentioned above, there are seven distinct types of feathers known among extant birds, ranging from simple bristles to asymmetrical flight feathers, and therefore, all of these qualify as “modern-looking feathers.” Haynes states that “if the feather is defined as filaments, then they appear in basal coelurosaurs such as Sinosauropteryx” (Haynes 2022, 293). It would seem that her definition would demand reclassifying substantial numbers of theropod dinosaurs, including the Tyrannosauroidea, Compsognathidae, and others, as birds. We do not believe this is Haynes’ intention, and invite her to clarify whether a) the filamentous integument seen in Sinosauropteryx, Yutyrannus, and other coelurosaurian theropods is homologous to feathers or not; and b) if so, by what other characteristics should “bird’’ be defined?
Second, if only pennaceous feathers qualify as sufficiently “modern-looking,” then Haynes’ mention of their presence in maniraptoran dinosaurs (Haynes 2022, 293) would require these dinosaurs to be birds. Maniraptora non-controversially includes the Alvarezsauroidea, Therizinosauria, Oviraptorosauria, Troodontidae, Dromaeosauridae, and Scansoriopterygidae. Clear pennaceous feathers have been found in each of these groups save the Alvaresauriodea and Therizinosauria, whose members display more tuft-like feathers. Additionally, the Ornithomimosauria contains members with pennaceous feathers (van der Reest, Wolfe, and Curry 2016), so we can broaden the range of dinosaurs with such traits to the larger group called Maniraptoriformes (= Ornithomimosauria + Maniraptora). Calling these dinosaurs “birds’’ merely because they possess pennaceous and/or tufted feathers would go far beyond what any evolutionist has argued. For example, Therizinosaurus is a 10 m long, ponderous, herbivorous theropod with enormous scythe-like claws, and its close relative Beipiaosaurus displays featherlike integument with identifiable melanosomes (Li et al. 2014; Xu, Zheng, and You 2009). These lumbering beasts cannot be considered birds in any reasonable taxonomy. Likewise, the medium- and large-sized, sickle-clawed dromaeosaurids display ample evidence of feathers. This includes the presence of quill knobs (ulnar papillae) on the forearms of Velociraptor and specimens currently referred to Dakotaraptor, and pennaceous feather impressions along the arms and tail in Zhenyuanlong. None of these animals would be called “birds’’ by taxonomists, because possessing feathers does not, by itself, define birds.
So how are birds defined? As Haynes notes in her paper, there are numerous definitions of Aves and Avialae. Nonetheless, vertebrate paleontologists typically define Aves as inclusive of all living birds while the term Avialae is applied beginning with or near Archaeopteryx and is inclusive of the wide array of non-modern fossil bird groups plus modern birds (see Cau 2018) for a detailed discussion). While we are not advocating for a phylogenetic definition (as they are evolutionary in their construction), it is important to recognize that neither Aves nor Avialae are defined solely on the basis of possessing feathers because 1) feathers are known in numerous non-modern bird fossils classified outside of Aves; and 2) feathers are known among numerous dinosaurian taxa classified outside of Avialae.
Haynes, however, makes the following claim: “The presence of feathers is not the only characteristic but one of the main ones because feathers are central to the definition of aves [sic] since only birds are known for possessing feathers (Brush 1996, 2001; Lee and Spencer 1997; Paul 1988)” (Haynes 2022, 293). The citations given here are problematic. First, those published prior to the discovery of the feathered fossil taxa beginning in the late 1990s cannot adjudicate the current situation. Second, and more troubling, is that Brush argued precisely the opposite of Haynes’ claim, agreeing that Protarchaeopteryx and Caudiperyx were dinosaurs with “essentially modern contour and primary feathers” and that “the feathers on these specimens are symmetrical primaries and semiplume [feather types].” (Brush 2000, 632). Brush’s writings contemporary with Haynes’ citation are emphatic that feathers are not restricted to Aves (Brush 2000; Prum and Brush 2002).
This brings us to a curious situation. Because of the clear evidence of feathers on certain dinosaur fossils, a number of young-earth creationist authors have advocated for reclassifying these taxa as birds. We find these assignments to be biologically inconsistent, as they frequently separate members of the same taxonomic family into both dinosaur and bird categories. For example, Clarey (2015, 126–127) classifies Microraptor as a bird while other dromaeosaurids remain dinosaurs, and also classifies Scansoriopteryx as a bird. Thomas and Sarfati (2018) likewise raise questions concerning Microraptor’s status as a dinosaur, and they further suggest that Caudipteryx (and perhaps all of Oviraptorosauria) might be reassigned to birds. Cserhati, Thomas and Tay (2020) consider Caudipteryx a bird, even while their analysis clustered it with Incisivosaurus, another oviraptorid which they consider a dinosaur. Sarfati and Tay (2022) assign Microraptor, Anchironis, and Caudipteryx to birds but not other members of the Dromaeosauridae, Troodontidae, or Oviraptorosauria, respectively. The most conspicuous example comes from Menton (2018), who separated two skeletons of the same species, Ornithomimus edmontonicus, into “dinosaur” and “bird” based on pictures of their museum displays.
These claims are anatomically untenable. Descriptive paleontology is rigorous and precise, and it seems quite unlikely that well-trained paleontologists and comparative anatomists have mistakenly placed animals from two different classes into the same families (or even species!). The various proposals mentioned above lack the kind of methodical skeletal assessments that are necessary to make a rigorous anatomical argument. The sole unifying factor among them is the underlying assumption that any animal possessing feathers is, by definition, a bird. When this assumption is removed, it becomes clear that numerous, positively identified dinosaurs possess a wide range of feathers.
Finally, Haynes eschews a morphological definition of feathers, asserting instead that “feather means a complete and functional structure with no evolutionary stages and that only birds are known to possess” (emphasis original, Haynes 2022, 293). There are two significant problems with this. First, when combined with the definition of bird given earlier in the same paragraph, the two definitions become circular: only birds possess feathers, and feathers are only found among birds. By defining feathers as structures possessed only by birds, Haynes prejudges the question of whether anatomically recognized dinosaurs could possess genuine feathers. Second, we are uncertain precisely what Haynes means by “feather” in this context, since her definition does not include a description of any anatomical features. Without feather-specific information (including structure, composition, location, and development), we are left with nothing more than a biological structure unique to birds. This could include any number of features, such as the syrinx (the unique voice box that lets birds sing) or proximally fused metatarsals (midfoot bones). We realize that this is not what Haynes intends to argue, but we raise the issue to demonstrate that a precise definition for feathers is a prerequisite for determining whether or not such structures are found among dinosaurs.
The Anatomy of Archaeopteryx Tails and Pygostyles
Certain anatomical arguments are employed by Haynes in her claim that Archaeopteryx is best understood as a bird rather than a dinosaur. For instance, Haynes frequently states that Archaeopteryx possessed a pygostyle. She includes this in a list, ostensibly from Wellnhofer (2009), of eight features that Archaeopteryx shared with dinosaurs (Haynes 2022, 287). These claims are mistaken on two levels. First, a pygostyle is, by definition, composed of a short series of fused caudal (tail) vertebrae. A pygostyle is seen as the shortened bony tails of modern birds and some Mesozoic bird groups (for example, confuciusornithiforms, enantiornithines, etc.), which together comprise the group Pygostylia. In no way can the term pygostyle be applied to Archaeopteryx’s elongate tail, which consists of 21–23 unfused caudal vertebrae and most closely resembles the tails of Jeholornis and various maniraptoran theropods. Haynes has either misunderstood the nature of Archaeopteryx’s tail, misunderstood the term pygostyle, or is redefining pygostyle as the opposite of its meaning. Second, Wellnhofer has never referred to Archaeopteryx’s tail as a pygostyle, but asserts the exact opposite: “There is no fusion of the terminal caudals; no pygostyle is developed” (Wellnhofer 2009, 127). Furthermore, nowhere in Wellnhofer (2009) is the list of eight features presented by Haynes, and the order of Wellnhofer’s descriptive writing is altogether different from the list provided by Haynes. Wellnhofer explores in great detail how Archaeopteryx compares with theropods using the anatomical sequence employed by organismal biologists and paleontologists when describing a new species of vertebrate: skull and mandible, vertebral column, ribs and gastralia, pectoral girdle, forelimbs, pelvic girdle, and hind limbs (Haynes 2022, 162–166). Haynes’ list does not follow this pattern. Given the substantial differences in content, order, and the erroneous attribution of a pygostyle, it is difficult to see how Haynes compiled her list from Wellnhofer (2009).
In her fig. 6 illustrating tail bones from Archaeopteryx, various theropod dinosaurs, and modern birds, Haynes identifies a “rodlike portion of the tail acting as pygostyles’’ in Archaeopteryx, Microraptor, Sinornithosaurus, Anchiornis, and Caudipteryx. Among these taxa, only Caudipteryx has a pygostyle composed of fused distal caudal vertebrae, and its morphology is quite different from modern birds (Qiang et al. 1998). The only other dinosaurs known to have a pygostyle are Nomingia (Barsbold et al. 2000; likely equivalent to Elmisaurus rarus, Funston et al. 2021) and Similicaudipteryx (He, Wang, and Zhou 2008), although pygostyle-like fused vertebrae do occur at the ends of the tails of the therizinosaur Beipiaosaurus (Xu et al. 2003) and the bizarre ornithomimosaur Deinocheirus (Lee et al. 2014). Microraptor and Sinornithosaurus possess elongate tails that are stiffened into a rod by hyper-elongated prezygapophyses and haemal arches (also called chevrons). This arrangement, called a caudotheca (Senter et al. 2012), is found widely among the dromaeosaurids such as Bambiraptor, Deinonychus, Velociraptor, and Zhenyuanlong, as well as in rhamphorhynchid pterosaurs (Persons and Currie 2012). The caudotheca is altogether distinct from a pygostyle, as there is no fusion of the bones, but rather an interweaving of the bony processes among the vertebrae. We are unclear how the caudotheca is “acting as a pygostyle,” since the pygostyle anchors tail feathers while the caudotheca has long been recognized as a balancing structure for these bipedal theropod dinosaurs (Ostrom 1969).
No caudotheca is developed in Archaeopteryx, which possesses only mildly enlarged prezygapophyses and haemal arches that do not extend beyond the vertebral centrum, but which results in the distal two-thirds of its tail being stiff or somewhat elastic (Wellnhofer 2009). This condition is also seen in some dromaeosaurids (for example, Achillobator, Buitreraptor, and Utahraptor) and troodontids (Makovicky, Apesteguía, and Agnolín 2005; Senter et al. 2012). Anchiornis possess neither fused vertebrae nor a caudotheca, so its tail is certainly not “rodlike” at all, but flexible (Lindgren et al. 2015). Importantly, the comparative taxa presented by Haynes are all members of various dinosaurian families within the Maniraptora (Dromaeosauridae, Troodontidae, and Caudipteridae), and none of the tails among this group are remotely similar to those of the birds presented in her fig. 6, all of which possess actual pygostyles (goose, juvenile hoatzin, and ostrich).
Also in fig. 6, Haynes offers a criterion of ten or fewer caudal vertebrae before a “transition point” to a pygostyle or rodlike tail as a way to classify Archaeopteryx and the maniraptorans mentioned above as birds. However, the nature of this purported transition point is undefined, unexplained, and, we believe, a highly questionable approach to delineating dinosaurs and birds. Even if Haynes’ proposed transition point was a legitimate character, why would this single feature be more important than other characters used to distinguish birds from dinosaurs? Why could we not separate dinosaurs and birds by the presence or absence of clawed digits in adults or the presence or absence of true teeth? Indeed, if we considered the pectoral muscles used in flight, we would find that Archaeopteryx shares more in common with coelurosaur dinosaurs than with Confuciusornis and other birds (Pittman et al. 2022). Alternatively, a recent discovery of preserved intestinal remains in a feathered dromaeosaurid show that dromaeosaurids had more in common with coelurosaurian dinosaurs than modern birds in their alimentary canals (Wang et al. 2022). Why should the number of caudal vertebrae be more vital to classification than dentition, wing musculature, or digestive anatomy? After all, the number of tail vertebrae are highly variable, and many animals have shorter or longer tails than incredibly close relatives (for example, uakaris, bobcats, etc.). Even individuals of the same species can vary in the number of caudal vertebrae: as noted above, specimens of Archaeopteryx possess between 21 and 23 caudals. Such traits are justifiably considered minimally informative among taxonomists.
It appears, then, that Haynes assigns a number of dinosaurs to birds: Microraptor, Sinornithosaurus, Anchiornis, and Caudipteryx. Their blue coloration in fig. 6 is also employed in the extant birds, and contrasted with the green coloration given to several theropod tails lower in the figure. There is no further discussion of the figure, but it appears that her classification assignments follow those of Clarey (2015), Thomas and Sarfati (2018) and Cserhati, Thomas, and Tay (2020) in splitting apart various theropod families into dinosaur and bird members, and Haynes adds to this list by assigning Sinornithosaurus (a dromaeosaurid) to birds. We find Haynes’ argument that these dinosaurs should be categorized as birds unjustified given the well-defined skeletal definitions of these dinosaurian groups, the incorrect assertion of pygostyles or pygostyle-like tails among these taxa, and the questionable argument for an (undefined) transition point along the tail. A far more parsimonious conclusion is that a substantial number of dinosaurs possess true feathers.
The Hoatzin, Archaeopteryx, and Character States
Haynes’s reference to a few analogous structures between the South American hoatzin and cows is certainly not evidence that the many homologous structures in Archaeopteryx and dinosaurs should be ignored or downplayed. The hoatzin’s expanded crop ferments plant material and so does a cow’s rumen, yet the anatomy of these two structures are entirely different (Godoy-Vitorino et al. 2010). This means that the organs are analogous (different anatomical structures performing a similar function) rather than homologous (composed of the equivalent anatomical structures). Likewise, the presence of wing claws in hoatzins does not allow the homologous structures in Archaeopteryx and dinosaurs to be dismissed. Hoatzin wing claws are not unique among birds; the presence of one or two wing claws (and rarely a third, non-keratinized, non-ossified claw) is a relatively common feature among living birds, including parrots, ratites (for example, ostriches and cassowaries), and raptors (for example, great gray owls and red-tailed hawks; Fisher 1940; Nero and Loch 1984; Olsen, Ross, and Olsen 1987; Wein and Schwing 2017). Hoatzins and all other extant, flying birds that have wing claws possess the same basic type of hands/wings: a fusion of hand bones (the carpometacarpus) and 1–2 bones (phalanges) per finger, besides claws (if present). In contrast, Archaeopteryx had non-fused carpals and metacarpals and long, multiple-phalanx fingers tipped with fully ossified claws, much like Deinonychus (fig. 4).
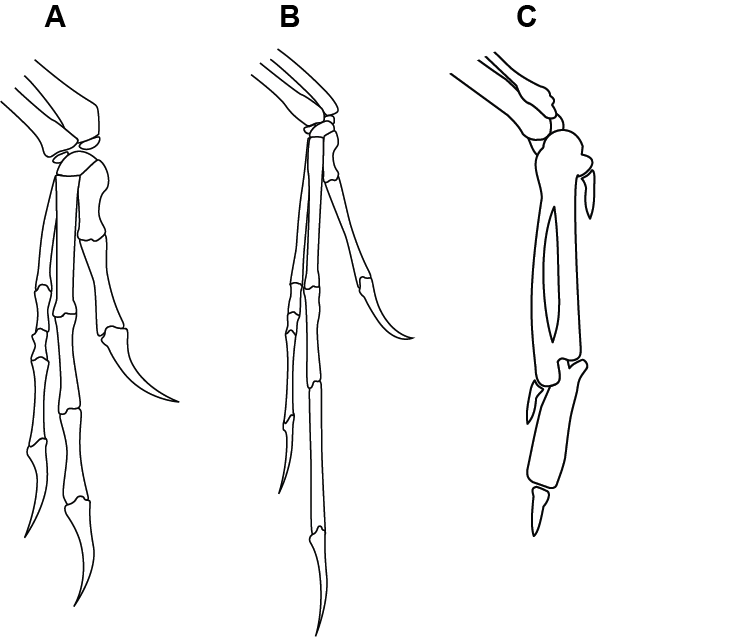
Fig. 4. Line drawings of the hand skeletons of Deinonychus (A), Archaeopteryx (B), and Passer domesticus (the house sparrow, C). Deinonychus and Archaeopteryx illustrations by John Conway. “The hands of Deinonychus (left) and Archaeopteryx (right) compared.” CC BY-SA 3.0. https://en.wikipedia.org/wiki/Deinonychus#/media/File:Archaeo-deinony_hands.svg. Sparrow illustration is modified from Retired electrician, “File: SwiftWingBones.png” https://commons.wikimedia.org/wiki/File:Bird_wing_skeleton._Passeriformis.svg,. CC0 1.0 DEED.
Haynes also refers to hoatzins as phylogenetic enigmas, but this merely concerns which bird group is most similar to hoatzins. No one questions the classification of hoatzins as birds or thinks it might be more closely related to cows or reptiles. Hoatzins are highly unusual among living birds, but the taxon is part of Neornithes (crown-group birds according to evolutionists). Thus, even according to evolutionary theory, the hoatzin is not considered any more “reptilian” than any other living bird. It is likely that hoatzins are in their own created kind, although research is necessary to evaluate this hypothesis.
The situation of Archaeopteryx and maniraptoran dinosaurs is unquestionably different, in that numerous anatomical traits seen in the specimens of Archaeopteryx show clear homology to theropod dinosaurs. Returning to the random selection of 20 characters from Table 1 above, we have included the character states for two ~100 kg maniraptoran theropods (Falcarius, a 4–5 m long therizinosaur, and Deinonychus, a 3.4 m long dromaeosaurid), Archaeopteryx, and the crow-sized Cretaceous bird Confuciusornis. These taxa are chosen for anatomical comparison to Archaeopteryx because their taxonomic status is uncontroversial (two decidedly terrestrial dinosaurs and one volant bird) and the substantially complete nature of their skeletal remains maximizes the number of verifiable character states available for comparison.
Tallying these taxa, we find that only one character state (#310) is definitely shared among all four taxa. Three additional character states (5, 32, and 77) are shared among Deinonychus, Archaeopteryx, and Confuciusornis but are unknown in Falcarius, while three character states (99, 345, and 763) are shared among Falcarius, Deinonychus, and Archaeopteryx but unknown in Confuciusornis. There are thus six character states in which we cannot determine if there is a difference among one of the four taxa.
Differences between Archaeopteryx and Confuciusornis are found in three characters: 80, 478, and 784. Character 80 codes for teeth on the maxilla. Archaeopteryx is coded as “0” (present) and Confuciusornis is coded as “1” (absent). Character 478 records the length of the skull as being below (Archaeopteryx) or above (Confuciusornis) 40% of the animal’s trunk length. Character 784 codes for the shape of the foremost teeth on the dentary, with those in Archaeopteryx coded as “1” (ziphodont, or blade-shaped, teeth), and Confuciusornis is coded as a “?”. While the “?” is normally reserved for unknown states due to incomplete or missing fossil material, it is used here as “inapplicable” because Confuciusornis has no teeth on its dentary bone and thus cannot be coded by a 0 or 1. The edentulous state of Confuciusornis’ dentary is already coded by character 217 (not selected in our random sample), so by assigning a “?” for character 478 and other tooth-related characters (of which there are several), the authors avoid having these irrelevant characters affect the analysis of edentulous taxa. This is one example of how care and caution are taken to produce a high-quality dataset.
In each of these three characters (80, 478, and 784), Archaeopteryx displays more in common with the maniraptoran dinosaurs Falcarius and Deinonychus than with the bird Confuciusornis. Additionally, we find that there are two character states in Table 20 that are different between Archaeopteryx and Falcarius (610 and 784), while there are zero definite differences between Archaeopteryx and Deinonychus. And though these 20 characters are only a sampling of the full dataset presented by Brusatte et al (2014; see their supplemental data), they are nonetheless fairly representative of the overall situation. This is why several statistical baraminological studies have seen Archaeopteryx frequently (but not always) cluster with dromaeosaurids (Doran et al. 2018; Garner, Wood, and Ross 2013; and McLain, Petrone, and Speights 2018). These animals show a striking degree of skeletal similarity, and this is based on homology, not analogy.
Skeletal Pneumaticity
Concerning pneumatization of the skeleton, we agree with Haynes that the best evidence so far points to its main role being a lightening of the skeleton in birds, pterosaurs, sauropods, and theropods (Farmer 2006), and that it is unclear what role (if any) pneumatization might play in respiration. However, Haynes appears to misunderstand the evidence for skeletal pneumaticity in these animals and how soft parts are inferred in fossil organisms. Haynes makes the argument that skeletal pneumatization in dinosaurs should not be taken as evidence for the presence of bird-like air sacs, and that the only reason scientists have argued this point is “to support the theory of the relationship between birds and dinosaurs” (Haynes 2022, 294). O’Connor (2006) recognized that claims of postcranial skeletal pneumaticity (PSP) in non-ornithodiran archosaurs (for example, crocodilians) were misidentified. Holes and cavities in bones are not automatic evidence for PSP, as they can be related to the presence of fat bodies or blood vessels. True PSP comes in the form of foramina or fossae connected to large internal chambers in bones (O’Connor 2006). O’Connor (2006) then proceeds to say that true PSP is found in theropods, sauropods, and pterosaurs and that, “Vertebral and costal pneumaticity in neotheropods supports the inference of cervical and abdominal air sacs in this group, in addition to pneumatization directly from the lung” (O’Connor 2006, 1222). Butler, Barrett, and Gower (2012) also find “unambiguous evidence of PSP . . . in bird-line (ornithodiran) archosaurs” (Butler, Barrett, and Gower 2012, 1). Haynes cites both of these references as evidence in favor of her position, when they—in fact—argue the complete opposite.
Beyond this discussion of PSP anatomy, Haynes’ argument that the only reason scientists would claim these structures were in some dinosaurs is because they believe dinosaurs evolved into birds reveals a seeming misunderstanding of biological similarity and comparative anatomy. Applications of comparative anatomy and physiology hold up whether someone believes in universal common descent or not. When we test medications on a rat and they induce liver failure, we assume the same may be true for a human even though we do not believe humans and rats share a common ancestor. We believe this because we, through scientific study, have realized that humans and rats are built along similar mammalian blueprints. We correctly recognize the homology of the radius and ulna in a bird’s wing and a manatee’s flipper even while we, as young-earth creationists, reject the argument of common ancestry among them. Being a creationist does not mean we reject homology. Rather, we reject the idea that homology can only be explained by common descent, and instead affirm homology as a reflection of God’s design plans. The most anatomically similar extant animals to dinosaurs and pterosaurs are crocodilians and birds. This is confirmed by genetic studies that consistently place birds closer to crocodilians than to any other animal (for example, Cotton and Page 2002; Fong and Fujita 2011; Zardoya and Meyer 1998). Thus, it is not unreasonable to look at bird and crocodilian soft tissue anatomy to make hypotheses about those structures not preserved in the fossil record. Of course there will be surprises, but comparison to living creatures helps ground our speculation. Acknowledging anatomical similarity is not the same as acknowledging evolutionary history, and to think or act in this manner is tacit agreement that similarity is evidence for evolutionary descent.
Miscellaneous Paleontological Issues
In addition to the above issues, there are some minor errors in Haynes (2022) that are nonetheless troubling to see in a paper on vertebrate paleontology. Haynes refers to Majungasaurus as a sauropod (the enormous, long-necked dinosaurs), when it is in fact a theropod (the bipedal, carnivorous dinosaurs). Haynes labels Sapeornis an enantiornithine in fig. 3, but it does not belong to this group (it is, instead, an omnivoropterygid—an extinct bird group outside of Pygostylia). Haynes also wrote that Heilmann propagated a view that helped to shape the idea of a relationship between Archaeopteryx and dinosaurs when, in fact, Heilmann argued in his 1926 book that birds were not descended from dinosaurs because dinosaurs lacked clavicles (Heilmann’s clavicle argument would later be disproved when clavicles were discovered on dinosaurs). Finally, Haynes states that the arched-back neck seen in Archaeopteryx specimens has been demonstrated to happen in hypersaline water. However, experimental research on this phenomena, called the opisthotonic death posture, has indicated it occurs in cool freshwater (Cutler et al. 2011), and other researchers previously could not replicate the posture in saltwater (Faux and Padian 2007). This topic is in dire need of further experimental research from creationists and would be an excellent taphonomy project.
Interpreting Scripture
Haynes states that God’s Word is clear and definitive (Haynes 2022, 297), and we agree. We also recognize, though, that interpreting Scripture is a human activity and thus open to misunderstanding and error. For this reason we pay careful attention to, and seek careful exposition of, the biblical text with an eye both to what it says and what it does not say. For instance, Haynes states “God created birds before He created dinosaurs, and not the contrary. They are different kinds created on different days of the Creation Week” (Haynes 2022, 297). This statement contains several errors:
- The term “kind” (Hebrew, mîn) is a rather generic term, and its meaning with regard to taxonomy is not specified in Genesis 1. Nonetheless it is constrained in certain ways. For example, on the third day of Creation week God created fruit trees according to their kinds (Genesis 1:11–12). This would imply that “fruit tree” is not itself a kind, but a general descriptor for many kinds of plants. The terms for “bird” and “dinosaur” are neither in the text (see point 2), nor would they likely constitute a kind of their own, as there are many kinds of flying creatures and many kinds of beasts.
- Haynes misallocates her taxonomic scheme to the terms used in Scripture. Genesis 1 does not use the word tsippor (“bird”), but rather the broader term oph, which refers to flying creatures generally and includes birds, bats, and flying insects (cf. Leviticus 11:13–21). Interestingly, Leviticus 11:16 lists the ostrich as oph, even though it does not fly, indicating that the term covers not just “flying creatures,” but also includes the non-flying members of an otherwise flying category in a similar way that the word “bird” does in English (both before and after formal taxonomy). We expect, alongside many creationists, that other flying vertebrates such as pterosaurs are also oph and would thus have been created on Day 5. If feathered maniraptoran dinosaurs were originally created as flying creatures, they would have been made on Day 5 as well, regardless of whether modern scholars call them “birds” or “dinosaurs.” Ground-dwelling feathered dinosaurs may have been oph as well (like the ostrich) or may have been “beasts” (ḥay) created on Day 6. Having some members of a large taxonomic group created on different days should be uncontroversial, since various mammal groups were created on either Day 5 or Day 6, such as bats (oph, flying creatures) and aardvarks (ḥay ‘ereṣ, beasts of the earth), respectively.
- The argument presented by Haynes does not help us understand whether birds are dinosaurs or not because those are not questions of origin but questions of classification. For the evolutionist, classification invariably represents origins, but for the creationist some classification is based on origin (for instance, grouping humans by family units as is commonly done in the Old Testament), whereas other classification reveals the broader design blueprints of our Creator (for example, animal vs. plant, vertebrate vs. invertebrate, etc.). When a creationist says there are similarities between birds and dinosaurs or that a particular creature is a bird or a dinosaur, they are not making claims of evolutionary origins. Haynes, along with other creationists, has made a false equivalence between noting similarities among birds and dinosaurs and believing birds evolved from dinosaurs.
Conclusion
Rather than Archaeopteryx’s position as a bird being “straightforward,” we find that the situation is more complex. Organismal biology and taxonomy are demanding fields of study that require careful attention to minute details. In paleontology, uncertainties are increased by the limited nature of the specimens as fossilized remains instead of as living organisms. Nonetheless, confidence in certain conclusions can be found when employing rigorous standards of comparative anatomy. We can reasonably conclude that a large number of theropod dinosaurs possess feathers or feather-like integument. This is a point that Haynes appears to grant (Haynes 2022, 293), although it is unclear if Haynes actually accepts the assignment of filamentous and pennaceous feathers found with these fossils as legitimate, and she also reclassifies some of these taxa as birds. We further conclude that the general morphology of Archaeopteryx is remarkably similar to maniraptoran dinosaurs (particularly dromaeosaurids), many of which are also known to possess pennaceous feathers. In our estimation, Haynes (2022) has come to erroneous taxonomic conclusions based on misunderstandings on cladistics, statistical baraminology, comparative anatomy, dinosaurs, birds, and the interpretation of Genesis 1. These misunderstandings have led her to conclusions stated with far more certainty than is justified by the available evidence.
Despite these disagreements, we wish to make it abundantly clear that we agree with Haynes on all of the crucial issues pertaining to Scripture and young-earth creation. We agree that Scripture is inspired, infallible, and inerrant. We agree that the text of Genesis 1–2 explains the origins of the creatures we have today, with God creating different kinds of animals on Days 5 and 6 of Creation week and that these are earth-rotational days. We agree that the fossils of the organisms explored in our papers were produced during the year-long catastrophe of the Flood. Indeed, our agreement on these and many other issues are vastly more important, such that they should dwarf any disagreements we have over dinosaurs, feathers, and birds. This is not an issue of doctrine or Scripture’s authority, but rather a debate about the empirical data from the fossil record and its proper scientific interpretation. We recognize that Christians have the freedom to explore and take different scientific and philosophical positions so long as those positions do not contradict the clear teachings of Scripture. As we have demonstrated in this paper, our arguments that certain dinosaurs possessed feathers and that Archaeopteryx shares numerous traits with dinosaurs are based squarely on morphological data and are not “due to reliance on the assumptions of the evolutionary worldview” (Haynes 2022, 297). The creationist discussion on feathered dinosaurs, rather than being a creation/evolution debate, is actually a specific form of the ages-old functionalist/structuralist debate in biology (see, for example, Amundson 2007; Appel 1987; Aristotle c. 350 B.C.; Cuvier 1830; Gould 2002; Owen 1849). It is unwise for creationists to claim their philosophical or scientific position is the biblical position when, in fact, multiple positions are compatible with Scripture.
As creationists, we must be willing to evaluate the data from nature on its own terms, regardless of whether a discovery is made by a creationist or an evolutionist. Our goal as scientists is to understand God’s world so that we may grasp a more accurate picture of who God is and what He has made, and in so doing give glory to God and proclaim him to those we meet. We will necessarily disagree with each other as we seek to accomplish this task in the sciences, for we are ignorant and fallible creatures, but above all we must conduct ourselves personally and professionally with behavior that glorifies our heavenly Father.
Acknowledgements
We would like to thank several individuals who gave valuable input in the writing of this manuscript including J. McLain and D. Okonowski. We would also like to thank our two anonymous reviewers and our editor whose comments were helpful in revising our paper.
See Dr. Gabriela Haynes’ accompanying response.
References
Amundson, Ron. 2007. “Richard Owen and Animal Form.” In On the Nature of Limbs: A Discourse. Edited by R. Amundson, XV–LI. Chicago, Illinois: University of Chicago Press.
Appel, Toby A. 1987. The Cuvier-Geoffroy Debate: French Biology in the Decades before Darwin. Oxford, United Kingdom: Oxford University Press.
Aristotle. c. 350 B.C. On the Parts of Animals. In The Basic Works of Aristotle. Edited by Richard McKeon, 643–664. New York, New York: Random House.
Barsbold, Rinchen, Halszka Osmólska, Mahito Watabe, Philip J. Currie, and Khishigjaw Tsogtbaatar. 2000. “A New Oviraptorosaur (Dinosauria, Theropoda) From Mongolia: The First Dinosaur with a Pygostyle.” Acta Palaeontologica Polonica 45, no. 2: 97–106.
Brusatte, Stephen L., Graeme T. Lloyd, Steve C. Wang, and Mark A. Norell. 2014. “Gradual Assembly of Avian Body Plan Culminated in Rapid Rates of Evolution Across the Dinosaur-Bird Transition.” Current Biology 24, no. 20 (20 October): 2386–2392.
Brusatte, S.L., G.T. Lloyd, S.C. Wang, and M.A. Norell. 2015. Data from: Gradual assembly of avian body plan culminated in rapid rates of evolution across dinosaur-bird transition. Dryad, Dataset, https://doi.org/10.5061/dryad.84t75.
Brush, A. H. 1996. “On the Origin of Feathers.” Journal of Evolutionary Biology 9, no. 2 (March): 131–140.
Brush, Alan H. 2000. “Evolving a Protofeather and Feather Diversity.” American Zoologist 40, no. 4 (August): 631–639.
Brush, A. H. 2001. “The Beginnings of Feathers.” In New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom. Edited by Jacques Gauthier and Lawrence F. Gall, 171–179. New Haven, Connecticut: Peabody Museum of Natural History, Yale University.
Butler, Richard J., Paul M. Barrett, and David J. Gower. 2012. “Reassessment of the Evidence For Postcranial Skeletal Pneumaticity in Triassic Archosaurs, and the Early Evolution of the Avian Respiratory System.” PLoS ONE 7, no. 3 (March 28): e34094.
Cau, Andrea. 2018. “The Assembly of the Avian Body Plan: A 160-Million-Year Process.” Bollettino della Società Paleontologica Italiana 57, no. 1 (June): 1–25.
Cau, Andrea, Tom Brougham, and Darren Naish. 2015. “The Phylogenetic Affinities of the Bizarre Late Cretaceous Romanian Theropod Balaur bondoc (Dinosauria, Maniraptora): Dromaeosaurid or Flightless Bird?” PeerJ 3 (June 18): e1032.
Clarey, Tim. 2015. Dinosaurs: Marvels of God’s Design. Green Forest, Arkansas: Master Books.
Clausen, C., and M. A. McLain. 2021. “Interpreting Confusing Results in Pterosaur Baraminology Research.” Journal of Creation Theology and Science Series B: Life Sciences 11: 2.
Cotton, James A., and Roderic D. M. Page. 2002. “Going Nuclear: Gene Family Evolution and Vertebrate Phylogeny Reconciled.” Proceedings of the Royal Society of London B 269, no. 1500 (August 7): 1555–1561.
Cserhati, Matthew, Brian Thomas, and Joel Tay. 2020. “Hierarchical Clustering in Dinosaur Baraminology Studies.” Journal of Creation 34, no. 3 (December): 53–63.
Cutler, Alicia A., Brooks B. Britt, Rodney Scheetz, and Joshua Cotton. 2011. “The Opisthotonic Death Pose as a Function of Muscle Tone and Aqueous Immersion.” Journal of Vertebrate Paleontology 31 (January): 95.
Cuvier, G. 1830. “Considérations sur les Mollusques, et en Particulier sur les Céphalopodes.” Annales des Sciences Naturelles 19: 241–259.
DeWitt, David A., Anne Habermehl, and David Menton. 2010. “Baraminological Analysis Places Homo habilis, Homo rudolfensis, and Australopithecus sediba in the Human Holobaramin: Discussion. Answers Research Journal 3 (August 25): 153–158. https://answersresearchjournal.org/human-holobaramin-discussion/.
Doran, Neal A., Matthew McLain, Natalie Young, and Adam Sanderson. 2018. “The Dinosauria: Baraminological and Multivariate Patterns.” In Proceedings of the International Conference on Creationism. Edited by J. H. Whitmore, 404–457. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Farmer, C. G. 2006. “On the Origin of Avian Air Sacs.” Respiratory Physiology and Neurobiology 154, nos. 1–2 (November): 89–106.
Faux, Cynthia Marshall, and Kevin Padian. 2007. “The Opisthotonic Posture of Vertebrate Skeletons: Postmortem Contraction or Death Throes?” Paleobiology 33, no. 2 (Spring): 201–226.
Fisher, Harvey I. 1940. “The Occurrence of Vestigial Claws on the Wings of Birds.” American Midland Naturalist 23, no. 1 (January): 234–243.
Fong, Jonathan J., and Matthew K. Fujita. 2011. “Evaluating Phylogenetic Informativeness and Data-Type Usage for New Protein-Coding Genes Across Vertebrata.” Molecular Phylogenetics and Evolution 61, no. 2 (November): 300–307.
Foth, Christian, Helmut Tischlinger, and Oliver W. M. Rauhut. 2014. “New Specimen of Archaeopteryx Provides Insights into the Evolution of Pennaceous Feathers.” Nature 511, no. 7507 (2 July): 79–82.
Funston Gregory F., Philip J. Currie, Chinzorig Tsogtbaatar, and Tsogtbaatar Khishigjav. 2021. “A Partial Oviraptorosaur Skeleton Suggests Low Caenagnathid Diversity in the Late Cretaceous Nemegt Formation of Mongolia.” PLoS ONE 16, no. 7 (July 12): e0254564. https://doi.org/10.1371/journal.pone.0254564.
Garner, Paul A. 2009. “Evolving Christian Views of Species.” In Genesis Kinds: Creationism and the Origin of Species, CORE Issues in Creation 5 (January 16). Edited by Todd Charles Wood and Paul A. Garner, 7–29. Eugene, Oregon: Wipf & Stock.
Garner, Paul A., Todd C. Wood, and Marcus Ross. 2013. “Baraminological Analysis of Jurassic and Cretaceous Avialae.” In Proceedings of the International Conference on Creationism 7. Edited by Mark Horstemeyer, article 7.
Godoy-Vitorino, Filipa, Katherine Goldfarb, Eoin L. Brodie, Maria A. Garcia-Amado, Fabian Michelangeli, and Maria G. Dominguez-Bello. 2010. “Developmental Microbial Ecology of the Crop of the Folivorous Hoatzin.” The ISME Journal 4, no. 5 (4 February): 611–620. https://doi.org/10.1038/ismej.2009.147.
Gould, Stephen Jay. 2002. The Structure of Evolutionary Theory. Cambridge, Massachusetts: Belknap Press.
Haynes, Gabriela. 2022. “The Debate Over Classification of Archaeopteryx as a Bird.” Answers Research Journal 15 (September 14): 285–300. https://answersresearchjournal.org/dinosaurs/debate-classification-archaeopteryx-bird/.
He, Tao, Xiaolin Wang, and Zhong-He Zhou, Z.-H. 2008. A new genus and species of Caudipterid dinosaur from the Lower Cretaceous Jiufotang Formation of western Liaoning, China. Vertebrata PalAsiatica 46, no. 3: 178–189.
Huxley, T. H. 1870. “Further Evidence of the Affinity Between the Dinosaurian Reptiles and Birds.” Quarterly Journal of the Geological Society 26, nos. 1–2 (February): 12–31.
Jeanson, Nathaniel T. 2022. Traced: Human DNA’s Big Surprise. Green Forest, Arkansas: Master Books.
Lee, M. Y. S., and P. S. Spencer. 1997. “Crown-Clades, Key Characters and Taxonomic Stability: When is an Amniote not an Amniote?” In Amniote Origins: Completing the Transition to Land. Edited by Stuart S. Sumida and Karen L. M. Martin, 61–84. San Diego, California: Academic Press.
Lee, Yuong-Nam, Rinchen Barsbold, Philip J. Currie, Yoshitsugu Kobayashi, Hang-Jae Lee, Pascal Godefroit, François Escuillié, and Tsogtbaatar Chinzorig. 2014. “Resolving the Long-Standing Enigmas of a Giant Ornithomimosaur Deinocheirus mirificus.” Nature 515, no. 7526 (22 October): 257–260.
Li, Quanguo, Julia A. Clarke, Ke-Qin Gao, Chang-Fu Zhou, Qingjin Meng, Daliang Li, Liliana D’Alba, and Matthew D. Shawkey. 2014. “Melanosome Evolution Indicates a Key Physiological Shift Within Feathered Dinosaurs.” Nature. 507, no. 7492 (12 February): 350–353.
Lindgren, Johan, Peter Sjövall, Ryan M. Carney, Aude Cincotta, Per Uvdal, Steven W. Hutcheson, Ola Gustafsson et al. 2015. “Molecular Composition and Ultrastructure of Jurassic Paravian Feathers.” Scientific Reports 5: 13520. https://doi.org/10.1038/srep13520.
Linné, Carl von. 1806. Systema Naturae. Translated by William Turton. 10th ed. London, United Kingdom: Lackington, Allen, and Co.
Makovicky, Peter J., Sebastián Apesteguía, and Federico L. Agnolín. 2005. “The Earliest Dromaeosaurid Theropod from South America.” Nature 437, no. 7061 (13 October): 1007–1011.
McLain, Matthew, Matt Petrone, and Matthew Speights. 2018. “Feathered Dinosaurs Reconsidered: New Insights from Baraminology and Ethnotaxonomy.” In Proceedings of the International Conference on Creationism 8. Edited by John H. Whitmore, 472–515. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Menton, David. 2018. “Did Dinosaurs Evolve into Birds?” Answers in Depth (September 7). https://answersingenesis.org/dinosaurs/feathers/did-dinosaurs-evolve-into-birds/.
Nero, Robert W., and Steven L. Loch. 1984. “Vestigial Wing Claws on Great Gray Owls, Strix nebulosa.” The Canadian Field-Naturalist 98, no. 1 (January–March): 45–46.
Nesbitt, Sterling J. 2011. “The Early Evolution of Archosaurs: Relationships and the Origin of Major Clades.” Bulletin of the American Museum of Natural History 352 (29 April): 1–292.
O’Connor, Patrick M. 2006. “Postcranial Pneumaticity: An Evaluation of Soft-Tissue Influences on the Postcranial Skeleton and the Reconstruction of Pulmonary Anatomy in Archosaurs.” Journal of Morphology 267, no. 10 (October): 1199–1226.
Olsen, Penny D., Tony Ross, and Jerry Olsen. 1987. “Vestigial Wing Claws in Australian Birds of Prey.” Australian Bird Watcher 12, no. 1 (January): 20–21.
Ostrom, John H. 1969. “A New Theropod Dinosaur From the Lower Cretaceous of Montana.” Postilla 128 (25 February): 1–17
Ostrom, John H. 1976. “Archaeopteryx and the Origin of Birds.” Biological Journal of the Linnean Society 8, no. 2 (June): 91–182.
Owen, Richard. 1849. On the Nature of Limbs: A Discourse. London, United Kingdom: John Van Voorst.
Owen, Richard. 1863. “On the Archaeopteryx of von Meyer, With a Description of the Fossil Remains of a Long-Tailed Species, From the Lithographic Stone of Solenhofen.” Philosophical Transactions of the Royal Society of London 153 (31 December): 33–47.
Paul, Gregory S. 1988. Predatory Dinosaurs of the World: A Complete Illustrated Guide. New York, New York: Simon and Schuster.
Persons, W. Scott IV, and Philip J. Currie. 2012. “Dragon Tails: Convergent Caudal Morphology in Winged Archosaurs.” Acta Geologica Sinica 86, no. 6 (December): 1402–1412.
Pittman, Michael, Thomas G. Kaye, Xiaoli Wang, Xiaoting Zheng, Alexander Dececchi, and Scott A. Hartman. 2022. “Preserved Soft Anatomy Confirms Shoulder-Powered Upstroke of Early Theropod Flyers, Reveals Enhanced Early Pygostylian Upstroke, and Explains Early Sternum Loss.” Proceedings of the National Academy of Sciences 119, no. 47 (November 14): e2205476119.
Prum, Richard O., and Alan H. Brush. 2002. “The Evolutionary Origin and Diversification of Feathers.” Quarterly Review of Biology 77, no. 3 (September): 261–295.
Qiang, Ji, Philip J. Currie, Mark A. Norell, and Ji Shu-An. 1998. “Two Feathered Dinosaurs From Northeastern China.” Nature 393, no. 6687 (25 June): 753–761.
Saber, Ashraf Sobhy, and A. Hassanin. 2014. “Some Morphological Studies on the Wing and Foot of the Southern Cassowary (Casuarius casuarius).” Journal of Veterinary Anatomy 7, no. 2 (October):17–32.
Sanders, Harry, and Matthew Cserhati. 2022. “Statistics, Baraminology, and Interpretations: A Critical Evaluation of Current Morphology-Based Baraminology Methods.” Creation Research Society Quarterly 58 (Winter): 175–192.
Sanders, R. W. 2016. “Evidence for Holobaraminic Status of the Verbenaceae (Verbena Family).” Journal of Creation Theology and Science, Series B: Life Science 6 (September 26): 81–90.
Sarfati, Jonathan, and Joel Tay. 2022. Titans of the Earth, Sea, and Air. Powder Springs, Georgia: Creation Book Publishers.
Senter Phil, James I. Kirkland, Donald D. DeBlieux, Scott Madsen, and Natalie Toth. 2012. “New Dromaeosaurids (Dinosauria: Theropoda) From the Lower Cretaceous of Utah, and the Evolution of the Dromaeosaurid Tail.” PLoS ONE (May 15): e36790.
Simões, Tiago R., Oksana Vernygora, Illaria Paparella, Paulina Jimenez-Huidobro, and Michael W. Caldwell. 2017. “Mosasauroid Phylogeny Under Multiple Phylogenetic Methods Provides New Insights on the Evolution of Aquatic Adaptations in the Group.” PLoS One 12 (May 3): e0176773.
Surtees, Marc. 2021. “Is It a Bird? A Critical Analysis of Feathered Fossils.” e-Origins: Journal of the Biblical Creation Trust 3: 12–19.
Thomas, Brian, and Jonathan Sarfati. 2018. “Researchers Remain Divided Over ‘Feathered Dinosaurs’.” Journal of Creation 32, no. 1 (April): 121–127.
van der Reest, Aaron J., Alexander P. Wolfe, and Philip J. Curry. “A Densely Feathered Ornithomimid (Dinosauria: Theropoda) From the Upper Cretaceous Dinosaur Park Formation, Alberta, Canada.” Cretaceous Research 58 (March): 108–117.
Wagner, A. 1862. “On a New Fossil Reptile Supposed to be Furnished with Feathers.” The Annals and Magazine of Natural History 3, no. 52 (April): 261–267.
Wang, Xuri, Andrea Cau, Bin Guo, Feimin Ma, Gele Qing, and Yichuan Liu. 2022. “Intestinal Preservation in a Birdlike Dinosaur Supports Conservatism in Digestive Canal Evolution Among Theropods.” Scientific Reports 12 (19 November): 19965.
Wein, Amelia. and Raoul Schwing. 2017. “Claws on the wings of Kea Parrots (Nestor notabilis).” Notornis 64 (March): 31–33.
Wellnhofer, Peter. 2009. Archaeopteryx: The Icon of Evolution. Munich, Germany: Verlag Dr. Friedrich Pfeil.
Wood, Todd Charles. 2016. “Taxon Sample Size in Hominin Baraminology: A Response to O’Micks.” Answers Research Journal 9 (December 28): 369–372. https://answersresearchjournal.org/taxon-sample-size-in-hominin-baraminology-response-to-omicks/.
Wood, Todd Charles. 2021a. “Baraminology by Cluster Analysis: A Response to Reeves.” Answers Research Journal 14 (July 14): 283–302. https://answersresearchjournal.org/cluster-analysis-response-to-reeves/.
Wood, Todd. 2021b. “An Expanded Character Set For Evaluating the Phylogenetic Position of Homo floresiensis.” (September 1). Dryad, Dataset. https://doi.org/10.5061/dryad.905qfttm7.
Xu, X., and Y. Guo. 2009. “The Origin and Early Evolution of Feathers: Insights From Recent Paleontological and Neontological Data.” Vertebrata PalAsiatica 47, no. 4 (October): 311–329.
Xu, Xing, Xiaoting Zheng, and Hailu You. 2009. “A New Feather Type in a Nonavian Theropod and the Early Evolution of Feathers.” Proceedings of the National Academy of Sciences 106, no. 3 (January 20): 832–834.
Xu, Xing, Yennien Cheng, Xiaolin Wang, and Chunhsiang Chang. 2003a. “Pygostyle-like Structure From Beipiaosaurus (Theropoda, Therizinosauroidea) From the Lower Cretaceous Yixian Formation of Liaoning, China.” Acta Geologica Sinica 77, no. 3 (September): 294–298.
Xu, Xing, Zhonghe Zhou, Xiaolin Wang, Xuewen Kuang, Fucheng Zhang, and Xiangke Du. 2003b. “Four-Winged Dinosaurs From China.” Nature 421, no. 6921 (23 January): 335–340.
Xu, Xing, Philip Currie, Michael Pittman, Lida Xing, Qingjin Meng, Junchang Lü, Dongyu Hu, and Congyu Yu. 2017. “Mosaic Evolution in an Asymmetrically Feathered Troodontid Dinosaur With Transitional Features.” Nature Communications 8 (2 May): 14972. doi: 10.1038/ncomms14972.
Zardoya, Rafael, and Axel Meyer. 1998. “Complete Mitochondrial Genome Suggests Diapsid Affinities of Turtles.” Proceedings of the National Academy of Sciences 95, no. 24 (November 24): 14226–14231.
















