Research conducted by Answers in Genesis staff scientists or sponsored by Answers in Genesis is funded solely by supporters’ donations.
Abstract
Biosystematics is in great flux today because of the plethora of genetic research continually shedding light on organism relationships. Despite the large amount of data being published, the challenge is having enough knowledge about genetics to draw conclusions regarding the biological history of organisms and their taxonomy. Despite these uncertainties, an initial attempt to count and identify biblical kinds in amphibian orders Caudata and Gymnophiona were estimated using current information and several key assumptions and guidelines. They include focusing on monophyly based on morphological and genetic characters, maintaining taxonomic stability, relying on authors who demonstrate expertise in systematics, considering the usefulness and general acceptance of nomenclature, using hybridization data as evidence that organisms are of the same kind, identifying the cognitum, and using statistical baraminology as a tool to assess holistic continuity and discontinuity amongst and between organisms. With the above parameters, and current systematics data from extant amphibians, the initial conclusions suggest that Noah had 53 extant Caudate kinds and one extant Gymnophionan kind on the Ark.
Keywords: Ark Encounter, baraminology, biosystematics, Lissamphibian, Caudata, Gymnophiona, Caecilian
Introduction
Creation research is guided by God’s Word which is foundational to the scientific models that are built. As Christian scientists, we believe God has communicated important highlights of earth history, such as the worldwide Flood described in Genesis 6–9, and which is consistent with the geological data (Snelling 2009). The Flood of Noah has many implications that must be considered when building a creation model of biology. In light of these implications, The Ark Encounter Project has tasked creation researchers to investigate several questions, some of which include:
- What did God mean by kind when He told Noah to bring two of each and seven—sevens of clean animals on board (Turner 2009; Williams 1997)?
- How have organisms diversified from their Ark ancestors (Wood 2003)?
- How can the Ark kind be recognized from today’s organisms (Brophy and Kramer 2007, pp. 10–11; Lightner et al. 2011; Sanders and Wise 2003)?
- How many kinds were taken on board the Ark (Woodmorappe 1996)?
The purpose of this paper is to make an initial estimate of the identification and number of the kinds taken on board the Ark using all available information. Here I address extant amphibian Orders; Caudata (salamanders) and Gymnophiona (Caecilians or worm-like amphibians) and explain the rationale for my conclusions. Future papers will address the extant Anuran kinds (frogs and toads) and the extent sauropsids that include lizards, tuataras, crocodiles, snakes, and turtles.
The State of Biosystematics and Taxonomy Today
Biosystematics is the science of discovering, classifying, and organizing biological diversity. The science of identifying taxa and naming organisms is taxonomy. There is no universally accepted procedure for organism classification (Amphibiaweb 2013). Currently, these disciplines are in great flux as researchers are putting more importance on new genetic data being accumulated for phylogeny development and much is being changed accordingly. Therefore, how organisms are named and organized today may change tomorrow. Major sources for amphibian classification include; Blackburn and Wake (2011, pp. 38–54); Dubois (2005); Duellman (1999); Duellman and Trueb (1986); Frost (1985); Frost et al. (2006) and Pyron and Wiens (2011). Herpetologists at Amphibiaweb (2013), using these sources, have outlined the following criteria for their taxonomic recommendations:
- Of primary importance is to focus on monophyly and identify the clade consisting of species and their descendants based on morphological and genetic characters.
- Maintain stability as it pertains to the association of names and their taxa.
- Rely on authors who demonstrate expertise in systematics.
- Consider the usefulness and general acceptance of nomenclature by amphibian researchers.
- Focus on “tree” thinking rather than nested hierarchies.
Creation biologists differ in our assumptions in that we focus on “forest” thinking and are interested in how creatures have diversified from the originally created baramins and more specifically, the archetypes that left the Ark. Though there are overlaps with the above criteria, Lightner et al. (2011) outline the following guidelines, in descending priority, from a baraminological perspective:
- Biblical evidence suggests that living things reproduce after their kinds and therefore the ability to hybridize in extant creatures will be evidence that they are the same kind (Genesis 1 and 7).
- Identify the cognitum because God created His image bearers with the ability to group things together through human cognitive senses (Sanders and Wise 2003).
- Assess characters in order to determine holistic continuity and discontinuity amongst and between organisms using statistical baraminology (Wood 2006a; Wood 2006b).
For the purposes of this paper, all of the above considerations will be considered while incorporating the following precautions. Baraminologists tend to equate kinds with the family, and for many cases with good reason (Wood 2006a). However, we should carefully analyze the structures, behaviors, and physiologies of members of a putative kind and look at the genetic reasons why a certain member of a kind doesn’t have characters that the other members possess. When we better understand what mechanisms are involved in the production of the above characters, creation biologists will make more reasoned inferences about whether they were produced by post-Flood diversification through unknown genetic preprogrammed mechanisms or by direct creation. These considerations will affect the estimated number of kinds hypothesized. In the case of Caudates, there is a large variation within families and there will be an attempt to balance between lumping and splitting taxa. There will be cases where I will split because the genus seems to be the obvious cognitum and the reasons for variation are unknown. There will be other cases where I will lump and default to the Family or Order because they may be made of only one genus and/or there is hybridization, statistical baraminology and/or strong cognita that connects members.
| Suborder: Salamandroidea | Suborder: Cryptobranchoidea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behavior/Characters | Ambystomatidae | Amphiumidae | Salamandridae | Dicamptodontidae | Plethodontidae | Proteidae | Rhyacotritonidae | Sirenidae | Hynobiidae | Cryptobranchidae |
| Current Number of Species | 32 | 3 | 94 | 4 | 431 | 6 | 4 | 4 | 54 | 3 |
| Interspecific Hybridization | yes | yes | unknown | yes | yes | unknown | unknown | unknown | unknown | yes |
| Fertilization | internal | internal | internal | internal | internal | internal | internal | internal | external | external |
| Neoteny | varies/inducible | obligate | facultative | facultative | varies/inducible females | obligate | no neoteny | obligate | facultative | obligate |
| Parental Care of Eggs | female A. opacum | females | not reported | females | sometimes males | males or females | none | females | males | males |
| Average Total Length [TL] (cm) | 14 | 55 | 15 | 24 | 10 | 25 | 6 | 40 | 13 | 75 |
| Diploid Number (2n) | 28 | 28 | 22 or 24 | 28 | 26 or 28 | 38 | 26 | 46, 52, or 64 | 40, 56, 60 , or 62 | 60, 62, or 64 |
| Respiratory Structures | ||||||||||
| lungs | present | present | reduced | present | ABSENT | present | reduced | present | Present except Onychodactylus | present |
| ypsiloid | present | absent | present | present | absent | absent | present | absent | Present except Onychodactylus | present |
| larval gill slits | 3 pairs | 3 pairs | 4 pairs | 4 pairs | 3 or 4 pairs | 2 pairs | 4 pairs | 1 or 3 pairs | 4 pairs | 4 pairs |
| adult gills | variable | 1 pair gill slits | variable | variable | variable | present | none | external gills | none | absent |
| Skull Morphology | ||||||||||
| lacrimal bone | absent | absent | absent | present | absent | absent | present | absent | present | absent |
| premaxillae | separate | fused | single or separate | separate | paired or fused | separate | separate/paired | separate/paired | separate/paired | separated/paired |
| septomaxillae | present | absent | absent | present | present | absent | present | absent | present | absent |
| naso-labial grooves | absent | absent | absent | absent | present | absent | absent | absent | absent | absent |
| operculum | present | absent | present | present | present | absent | absent | absent | present or absent | absent |
| symphesial cartilage | absent | absent | absent | absent | absent | absent | absent | absent | absent | present |
| pterygoid | reduced | reduced | present | present | present in larvae | present | reduced | small | absent | present |
| Trunk and Vertebrae | ||||||||||
| limbs and toes | present | reduced | present | present | present | reduced | present | no hind limbs | present | present |
| scapula/caracoid bone of pectoral girdle | fused | reduced | fused | fused | fused | fused | fused | separate | fused | fused |
| ribs | bicapitate | bicapitate | bicapitate | bicapitate | bicapitate | bicapitate | bicapitate | bicapitate | unicapitate | unicapitate |
Superclass Tetrapoda, Class Amphibia
Amphibians are non-amniotic tetrapods which are four-legged vertebrates lacking an amniotic membrane that surrounds and builds the amniotic sac of reptile, bird, and mammal embryos. Amphibians also include members with reduced and/or absent legs. The word amphibian carries the idea of having two modes of existence or “two-lives” and refers to having both a water and land stage in their life cycle. The reality is that though many do have lives divided this way; many others do not (table 1). Subclass Lissamphibia are the extant amphibians and are further subdivided into three orders; Caudata, Anura, and Gymnophiona. Liss refers to their smooth, scaleless skin that is crucial for respiratory gas exchange and is a derived character. Other derived characters include bicuspid teeth, unique pedicellate teeth where the crown and base are made of dentine and have an uncalcified zone at the base, poison and mucous skin glands, a single element vertebral centrum, and reduced bones at the top of the skull. All are ectothermic and because their skin easily absorbs water, they don’t need to drink. Lissamphibians are also known for having the largest range of genome size variability among terrestrial vertebrates (Litvinchuk, Borkin, and Rosenov 2004). Currently it is estimated that there are over 7,000 species of Lissamphibians and at least 32% are being threatened with extinction for a host of reasons including habitat destruction (International Union for Conservation of Nature and Natural Resources 2012).
The tailed amphibians (Order: Caudata) include salamanders and newts and consists of two suborders (Salamandroidea with eight Families and Cryptobranchoidea with two) (table 1). All together they consist of 600 species or 9% of all amphibians (Amphibiaweb 2013). Diploid chromosome numbers (2N) and many other characters may vary both between and within Families (Larson, Wake, and Devitt 2006). Salamander bodies are elongate with most having four legs and a tail, and others having reduced and/or two absent appendages. Some species are obligate water creatures from egg to adult. Others must live on land for the length of their lifetime. Still others may begin as aquatic, gilled larvae and finish their lives as terrestrial, lunged, or lungless, adults. Salamanders are being extensively studied for their ability to regenerate full limbs after they are severed and this research has important implications for human medicine (Kragl et al. 2009).
Caecilians, Latin for caecus meaning blind and referring to their small or non-existent eyes, are in the Order Gymnophiona. Gymnophiona, Greek for gymnos (naked) and ophis (snake) comes from a time when members were called naked snakes because they did not have outer scales. They are probably the least known amphibian order and are fossorial (adapted for terrestrial digging and burrowing) or aquatic creatures characterized by their legless, elongate bodies that resemble earthworms to some and snakes to others (Pough et al. 2004, p. 8). This Order currently contains ten families, 191 species, and represents 3% of all Lissamphibians. What follows is a description of each delineated salamander and caecilian kind, average total lengths, various unique characters, and the rationale behind their baraminic classification.
Order Caudata—The Salamanders and Newts
A phenomenon common to salamanders is a process called neoteny. Neoteny (neotenic) is a word derived from the Latin referring to extended larval life, and is a process observed in many animals where adults retain juvenile characteristics. A type of neoteny found in salamanders is paedogenesis in which sexual reproduction can occur in individuals who retain a juvenile phenotype and do not fully metamorphose into terrestrial adults. These individuals are called paedomorphs. There are three types of neoteny; obligate neoteny, where all members retain their juvenile characteristics when they become adults. Obligate neotenes include all members of Amphiumidae, Sirenidae, Cryptobranchidae, and Proteidae. Inducible obligate neoteny happens when some members in Ambystomatidae and Plethodontidae can be induced to metamorphose into sexually mature terrestrial adults by manipulating the thyroid function in the laboratory or adding iodine to the environment. Facultative neoteny occurs when individuals may or may not be paedomorphic depending on environmental variables. This has been observed in Salamandridae, Dicamptodontidae, Hynobiidae, Plethodontidae, and Ambystomatidae (table 1).
Order: Caudata—Suborder Cryptobranchoidea
Cryptobranchoidea is a salamander suborder containing two Families, Cryptobranchidae and Hynobiidae. Among the major reasons they have been separated from the other eight families is because fertilization is external, like most fish, and lower jaw bones differ significantly. Consequently, they are called “primitive” salamanders.
Family Cryptobranchidae (Giant Water Salamander Kind)

Fig. 1. Giant water salamander kind. Source: Wikipedia http://en.wikipedia.org.
Cryptobranchidae consists of two genera (Andrias and Cryptobranchus) and three aquatic species. They have gills as larvae and lose them through partial metamorphosis when they reach adulthood. Though they have lungs, most of their respiration occurs by oxygen diffusion through skin and are therefore dependent on oxygen rich, turbulent streams in the wild (Jensen et al. 2008, p. 153). This Family contains the largest salamanders in the world with an average total length of 75 cm. The hellbender (Cryptobranchus alleganiensis) is restricted to eastern North America and has an average total length of 50 cm (Petranka 1998). The other two Asiatic species live in Asia. The Japanese giant salamander (Andrias japonicus) and the Chinese giant salamander (A. davidianus) can reach an average total length of 100 cm–120 cm and live 50 to 75 years in captivity (Larsen, Wake, and Devitt 2006). The Japanese giant salamander is revered and protected in Japan, but for many, the meat is considered a delicacy. To get around the protected status of A. japonicus, A. davidianus was introduced to Japan so that its meat could be sold at market (McNeil 2010). Consequently it is now considered an invasive species because A. davidianus is hybridizing with A. japonicus and there is mounting concern that the species “purity” of the Japanese giant salamander will be lost (McNeil 2010). From a biblical worldview, their hybridization ability connects them as the same kind.
Cryptobranchids have a unique caudate structure called a symphyseal cartilage which gives them the flexibility to suction feed with the left or right side of the mouth in their aquatic habitat (Amphibiaweb 2013). Adults are fully aquatic and reproduce by external fertilization, but they also have the ability to move across land (though rare) and gulp air. Other characters they share include; unicapitate ribs that have one head (facet) connecting to vertebrae, small lacrimal bones on the face are absent, prootic and exoccipitals skull bones are separate, fleshy skin folds are numerous, and the spiracle (external respiratory orifice in larvae) remains open in adults (Amphibiaweb 2013).
Fossils with very similar morphology as Andrias have been found in Cenozoic strata of the late Eocene to early Pliocene (Vasilyan, Böhme, and Winklhofer 2010) which suggests that this morphology may be a result of post-Flood diversification, if we assume the pre-Flood/post-Flood strata is denoted at the Cretaceous—Paleogene (K-Pg) boundary (Austin et al. 1994). Captive breeding and care has been done for many decades and it is possible that their archetype could have survived on the Ark. Therefore, because of their current systematics, having lungs, an ability to minimally maneuver on land, and the ability to breed in captivity, I include them on the Ark as the giant water salamander kind, until further research clarifies their taxonomic relationship with other caudates.
Family Hynobiidae (Asiatic Salamander Kind)

Fig. 2. Asiatic salamander kind. Source: Wikipedia http://en.wikipedia.org.
Family Hynobiidae is the other taxon of salamanders that fertilize eggs externally and current genetic data place them as a sister group of Cryptobranchidae (Amphibiaweb 2013). They are divided into two subfamilies, the Protohynobiinae (Protohynobius) and Hynobiinae which consists of the other nine genera. All together there are 54 species of small to medium size salamanders, endemic only to Asia, with an average total length of 12 cm. During the breeding season, males undergo a substantial increase in head width (Pough et al. 2004, p. 48). Larvae are aquatic, and even though facultative neoteny has been observed, most metamorphose into sexually mature terrestrial adults with eyelids, well developed lungs (except genus Onychodactylus that consists of two species of lungless terrestrial salamanders) and no gill slits. Adults in genera Batrachuperus, Liua, and Pachyhynobius are exceptions to the terrestrial mode and are aquatic (Larson, Wake, and Devitt 2006). As a cognitum, they are easily distinguished from all other Asian salamanders which suggest that they are a kind. There are no synapomorphies for this group, but they do share the following characters: septomaxillae (bones on the front of the upper jaw), lacrimals, vomerine teeth are not parallel to marginal teeth, and ribs are unicapitate (Amphibiaweb 2013). I did not find any records of interspecific hybridization in the family, though I would expect that it occurs. More research in this area would be helpful in clarifying family relationships. I include them as the Asiatic salamander kind on the Ark because of their strong cognita and well-developed adult lungs.
Order Caudata—Suborder Salamandroidea
The remaining eight Families described below belong to suborder Salamandroidea, or the “advanced” salamanders. Characters differ with Cryptobranchoidea in their jaw bone structures and reproductive behavior. Males produce spermatophores, which are little structures that house sperm, and they deposit them in their habitat. The male leads or coaxes an interested female over the deposited spermatophore. She then grasps it with the lips of her cloaca, and stores the sperm in an out-pocketing of her cloaca. As eggs pass through the cloaca they are internally fertilized. Females will deposit fertilized eggs either in water or on land, depending on the species. A few salamander species are viviparous and give birth to fully metamorphosed juveniles.
Family Ambystomatidae (Mole Salamander Kind)

Fig. 3. Mole salamander kind. Source: Wikipedia http://en.wikipedia.org.
The mole salamanders contain one genus, Ambystoma and 32 species. They are endemic from southern Canada to Mexico and are characterized by rounded heads and broad bodies with conspicuous costal grooves, or skin folds, along their sides (Petranka 1998, p. 35). Statistical baraminology suggests that this family is a monobaramin and interspecific hybridization confirms this (Brophy and Kramer 2007, pp. 10–11; Hennigan 2010; Petranka 1998 pp. 122–129). Hybrids may be diploid, triploid, tetraploid, or pentaploid. Polyploidy in vertebrates is unique and unisexual populations consisting of females are a result and may include the following species; A. texanum, A. tigrinum, A. laterale, and/or A. jeffersonianum. Average mole salamander total length is 14 cm and morphological characters connecting them include absent lacrimal bones, transverse oriented vomerine teeth (located on the roof of the mouth), and fused prootics, opisthotics and exoccipitals (small skull bones surrounding the inner ear) (Amphibiaweb 2013). The extinct genus Amphitriton is known from the upper Pliocene, and fossils of the extant genus Ambystoma are known from the lower Oligocene, through the Pleistocene, in North America (Heying 2003).
Many mole salamanders begin as aquatic larvae which may metamorphose into sexually mature terrestrial adults with lungs like the spotted salamander (Ambystoma maculatum). Ambystomatid larval characteristics include external gills, the presence of lateral line systems (sense organs used to detect underwater vibrations), and the absence of eyelids (Pough et al. 2004, p. 35). Some members are facultative neotenes or inducibly obligate neotenes. For example, the Axolotl (Ambystoma mexicanum), known for its importance in limb regeneration research (Kragl et al. 2009), is a neotene that is inducibly obligate and can metamorphose with thyroid hormones 3,5,3’-triiodothyronine (T3) and L-thyroxine (T4) in the lab (Page, Monaghan, and Walker 2009). T4 induced Axolotls will undergo metamorphic changes that include complete resorption of tail fins, gills, and dorsal ridges and will also experience reduced body mass and growth rate (Page, Monaghan, and Walker 2009). Other mole salamanders, depending on environmental variables, are facultative neotenes. I include the mole salamanders on the Ark because many have the ability to hybridize, they have a strong cognitum, and most adults are terrestrial with lungs. The data suggests, from both evolutionary and creationary interpretations, that ambystomatid paedomorphs are recently derived from a metamorphic ancestor (Page, Monaghan, and Walker 2009; Voss and Smith 2005) and therefore ambystomatid neoteny is probably a post-Flood phenomenon.
Family Dicamptodontidae (Large Land Salamander Kind)

Fig. 4. Large land salamander kind. Source: Wikipedia http://en.wikipedia.org.
Family Dicamptodontidae consists of one genus (Dicamptodon) and four species. Two extinct genera and the single extant genus are known from fossils in the North American Paleocene and the fossils of three extinct genera are known from the Paleocene and Miocene of Europe (Heying 2003). This Family contains the largest terrestrial salamanders with an average total length of 24 cm (Amphibiaweb 2013; Petranka 1998, pp. 145–156). Systematists have debated their taxonomic status for a long time (Petranka 1998, p. 145). Because of their strong cognita and other important characters, they used to be grouped with Ambystomatidae until recent years when they have been promoted to family status. I found one account of a breeder who has supposedly crossed Dicamptodon tenebrosus (Pacific Giant Salamander) with Ambystoma tigrinum (tiger salamander). However, because the claim is suspect, I have not found any reliable information that connects the two genera by hybridization and discount it until further evidence is gathered and verified.
Regarding interspecific hybridization within Dicamptodontidae, even though recent genetic comparisons between the Pacific giant salamander (D. tenebrosus) and the California giant salamander (D. ensatus) delineates them as separate species, they are connected by hybridization because they interbreed over a 4.7 km contact zone near Anchor Bay in Mendocino County, California (Amphibiaweb 2013). Gene flow is minimal and there is hybrid deficiency but the ability to interbreed places them within the same kind. All four species in Dicamptodontidae are endemic to the western United States and southwestern British Columbia and live in forested habitats with fast moving, permanent streams (Amphibiaweb 2013; Petranka 1998, p. 145). Eggs hatch into aquatic larvae and generally metamorphose into terrestrial adults with lungs, though facultative neoteny has been documented. Adults are nocturnal, known to eat small mammals, have a “barking” noise, and shared characters that include “M” shaped vomerine teeth, lacrimal bones, and marbled dorsal patterns (Amphibiaweb 2013). It is quite possible Dicamptodontidae and Ambystomatidae are part of the same mole salamander kind, especially because they share such strong cognita. However, keeping in mind the genetic and morphological data that distinguishes them, their current classification, and so that the number of kinds is not underestimated, I will denote them as the large land salamander kind until further research clarifies their relationship.
Family Rhyacotritonidae (Torrent Salamander Kind)

Fig. 5. Torrent salamander kind. Source: Wikipedia http://en.wikipedia.org/.
The four species and one genus (Rhyacotriton) in Rhyacotritonidae (torrent salamanders) are small salamanders with an average total length of 6 cm. No fossils have been reported for this family and they used to be classified with Ambystomatidae and later moved to Dicamptodontidae until 1992 when it was recommended that they should be separate because of differences in biochemical and morphological characters (Good and Wake 1992). They reside in extreme western North America and adults are semi aquatic producing fully aquatic larvae (Amphibiaweb 2013). Other shared characters include stocky build, eggs laid in cold water under rocks or in crevices, squared glands posterior to the vent, and a yellow green to bright yellow venter or belly (Amphibiaweb 2013). Their lungs are greatly reduced and they often have their vents resting in shallow water and/or always remain in saturated habitats. This is probably because Rhyacotriton species are probably the most desiccation intolerant salamanders known which suggests a high dependence on skin surfaces for oxygen diffusion (Amphibiaweb 2013). I was not able to locate hybrid data and much of the natural history for this family is unknown. Because of their strong cognita, the debate surrounding Rhyacotriton/Ambystoma systematics, and the desire not to underestimate the kinds, I include them in the Ark as the torrent salamander kind, until future researchers better understand and interpret the meaning of the genetic data in the context of their biosystematics and taxonomy.
Family Amphiumidae (Congo Salamander Kind)

Fig. 6. Congo salamander kind. Source: Wikipedia http://en.wikipedia.org.
The aquatic Congo salamanders consist of one genus, Amphiuma with three species having an average total length of 55 cm. They are endemic to the coastal plain of the southeastern United States (Amphibiaweb 2013; Jensen et al. 2008; Petranka 1998, p. 131). Eggs are usually laid on land under rocks or logs and near the water’s edge. Females coil around their 100 plus eggs and defend them until they hatch. Eggs are kept moist by their mother’s body and antibiotics produced by bacteria in her skin may protect them from harmful environmental fungi and bacteria (Jensen et al. 2008, p. 131). The length of the larval stage is variable and mostly unknown. Adults are paedomorphic and characters connecting them include laterally compressed tails, reduced pectoral and pelvic girdles, fused premaxillae (pair of small cranial bones on top of upper jaw), no eyelids, no tongue, open spiracle, lateral line systems, a single pair of gill slits, no external gills, reduced limbs, and lungs. They have variable numbers of toes per foot and this is one of the main characters that distinguish the three species. Members are the three-toed amphiuma (Amphiuma tridactylum), two-toed amphiuma (A. means), and one-toed amphiuma (A. pholeter) (Amphibiaweb 2013; Petranka 1998, p. 131). Amphiumidae contains a single extinct species in the genus Proamphiuma and is known from the Cretaceous (Larsen, Wake, and Devitt 2006).
Hybridization connects two of the three species (A. pholeter × A. means) in Louisiana with intermediate color patterns and toe numbers (Fontenot 2010). Some hybrid individuals had two toes on the front limbs and three on the back. These hybridization zones have highlighted the fact that amphiuman relationships are more complicated than researchers thought and much more genetic information is needed to shed light on their reproductive biology. I have included them on the Ark as the Congo salamander kind because much is unknown about their genetics and natural history, their partial reliance on land, having lungs that can gulp air when water is low in oxygen, and their ability to travel over land to disperse or hunt. If the habitat completely dries up they are able to burrow into the mud, surround their body with a mucous cocoon, and survive for as long as three years (Amphibiaweb 2013; Jensen et al. 2008, p. 150).
Family Plethodontidae (The Lungless Salamanders)
Family Plethodontidae is the largest salamander group with 27 genera and 431 species having an average total length of 10 cm. Their range extends from North, Central, and South America to Eurasia and they are subdivided into two subfamilies based on skeletal features and head muscles. Hemidactyliinae contains 20 genera and Plethodontinae has seven (Amphibiaweb 2013). Some members in Plethodontinae have aquatic larvae and other members have larvae that develop in eggs clustered under terrestrial rocks and logs. All salamanders in this family have no lungs and respiration takes place through their skin. Other shared characters include four fingers and five toes (with few exceptions), unique naso-labial grooves (grooves between each nostril and upper lip) used in chemoreception, absent pterygoids (structures on skull), absent lacrimals, and long bodies with up to 60 vertebrae (Amphibiaweb 2013). Fossils of six extinct genera are known from the North American lower Miocene to Pleistocene (Petranka 1998).
The family is quite diverse, for example, some can ballistically project their tongue to catch prey while others have web feet. Until further research sheds light on why they are so diverse, I default the kind to genus. It is probable that many will eventually be lumped into larger taxa in the future.
Subfamily Plethodontinae currently contains seven genera and 96 species.
- Genus Aneides (6 species)
Climbing Salamander kind

Fig. 7. Climbing salamander kind. Source: Source: Wikipedia http://en.wikipedia.org.
- Genus Desmognathus (21 species)
Dusky Salamander kind
Many are linked by interspecific hybridization.

Fig. 8. Dusky salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Ensatina (1 species)
Sword Salamander kind

Fig. 9. Sword salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Karsenia (1 species)
Korean Crevice Salamander kind
This was a surprise discovery because it is the only Asian plethodontid.

Fig. 10. Korean crevice salamander kind. Source: Berkeley University of California http://www.berkeley.edu/index.html. Photo: Rafe Brown.
- Genus Hydromantes (11 species)
Web Toed Salamander kind
Has web toes and can ballistically project tongue to capture prey.

Fig. 11. Web toed salamander kind. Source: California Herps http://www.californiaherps.com/index.html. Photo: Gary Nafis.
- Genus Phaeognathus (1 species)
Red Hills Salamander kind

Fig. 12. Red Hills salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Plethodon (55 species)
Woodland Salamander kind

Fig. 13. Woodland salamander kind. Source: Wikipedia http://en.wikipedia.org.
Subfamily Hemidactyliinae currently contains 20 genera and 335 species.
- Genus Batrachoseps (22 species)
Slender Salamander kind

Fig. 14. Slender salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Bolitoglossa (121 species)
Tropical Climbing Salamander kind

Fig. 15. Tropical climbing salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Bradytriton (1 species)
Guatemalan Salamander kind

Fig. 16. Guatemalan salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Chiropterotriton (12 species)
Splayfoot Salamander kind

Fig. 17. Splayfoot salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Cryptotriton (6 species)
Hidden Salamander kind

Fig. 18. Hidden salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Dendrotriton (8 species)
Bromeliad Salamander kind

Fig. 19. Bromeliad salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Theodore Papenfuss.
- Genus Eurycea (26 species)/Genus Haideotriton (1 species)
Brook Salamander kind

Fig. 20. Brook salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Gyrinophilus (4 species)
Spring Salamander kind

Fig. 21. Spring salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Hemidactylium (1 species)
Four-Toed Salamander kind

Fig. 22. Four-toed salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Ixalotriton (2 species)
Bounding Salamander kind

Fig. 23. Bounding salamander kind. Source: Cal Photos http://calphotos.berkeley.edu/about.shtml. Photo: Theodore Papenfuss.
- Genus Nototriton (16 species)
Moss Salamander kind

Fig. 24. Moss salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Nyctanolis (1 species)
Long-Limbed Salamander kind

Fig. 25. Long-limbed salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Oedipina (36 species)
Worm Salamander kind

Fig. 26. Worm salamander kind. Source: Smithsonian Tropical Research Institute http://www.stri.si.edu/. Photo: Marcos Guerra.
- Genus Parvimolge (1 species)
Tropical Dwarf Salamander kind
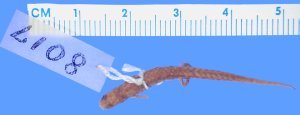
Fig. 27. Tropical dwarf salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Museum of Comparative Zoology, Harvard University.
- Genus Pseudoeurycea (49 species)
False Brook Salamander kind

Fig. 28. False brook salamander. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Pseudotriton (2 species)
Red-Mud Salamander kind

Fig. 29. Red-mud salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Stereochilus (1 species)
Many Lined Salamander kind

Fig. 30. Many lined salamander kind. Source: CalPhotos University of California, Berkeley http://calphotos.berkeley.edu/.
- Genus Thorius (24 species)
Minute Salamander kind

Fig. 31. Minute salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Sean Michael Rovito.
- Genus Urspelerpes (1 species)
Patch-Nose Salamander kind

Fig. 32. Patch-nose salamander kind. Source: Encyclopedia of Life http://eol.org. Photo: Todd Pierson.
Family Salamandridae (True Salamander and Newt Family)
Family Salamandridae includes the newts, subfamily Pleurodelinae, the “true” salamanders, subfamily Salamandrinae, and the spectacled salamanders, subfamily Salamandrininae. The average total length of the family is 13 cm (Amphibiaweb 2013). Their range occurs mostly in Eurasia, with some reaching northern Africa. Two genera represented in the United States and eastern Mexico are Notophthalmus and Taricha (Amphibiaweb 2013; Petranka 1998, p. 445). North American fossils are known from upper Oligocene, Miocene, and Pleistocene deposits and in Europe they are well represented in the Cenozoic strata (Petranka 1998, p. 445). Common characters shared include no nasolabial grooves, no costal grooves, two longitudinal rows of teeth extending far back into the mouth, a frontal squamosal arch on the skull, and vertebrae that are opisthocoelous or shaped so that their anterior surface is convex and their posterior surface is concave (Amphibiaweb 2013). Some true salamanders are viviparous and include Lyciasalamandra, Salamandra atra and S. lanzai (Larson, Wake, and Devitt 2006). They have several specific features that distinguish them from all other salamanders and many have warty skin that produces toxins (Petranka 1998, p. 445). Some start as aquatic larvae, become terrestrial juveniles, and return to water as adults. During breeding they exhibit sexual dimorphism and males often perform unique courtship dances (Amphibiaweb 2013). No relevant hybridization data was found and as with Plethodontidae, because of the large variation within the family, I default the kind to genus until further research sheds light on the mechanisms for these variations.
Subfamily Salamandrininae contains 1 genus and 2 species.
- Genus Salamandrina (2 species)
Spectacled Salamander kind

Fig. 33. Spectacled salamander kind. Source: Wikipedia http://en.wikipedia.org.
Subfamily Salamandrinae currently contains 4 genera and 18 species.
- Genus Chioglossa (1 species)
Gold-Striped Salamander kind

Fig. 34. Gold-striped salamander. Source: Wikipedia http://en.wikipedia.org.
- Genus Lyciasalamandra (10 species)
Greco-Turkish Salamander kind

Fig. 35. Greco-Turkish salamander kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Mertensiella (1 species)
Caucasian Salamander kind

Fig. 36. Caucasian salamander kind. Source: Georgian Biodiversity Database http://www.biodiversitygeorgia.net/index.php?taxon=Eucariota. Photo: David Tarkhnishvili.
- Genus Salamandra (6 species)
Fire Salamander kind

Fig. 37. Fire salamander kind. Source: Wikipedia http://en.wikipedia.org.
Subfamily Pleurodelinae has 16 genera and 77 species
- Genus Calotriton (2 species)
Spanish Brook Newt kind

Fig. 38. Spanish brook newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Cynops (8 species)
Firebelly Newt kind

Fig. 39. Firebelly newt kind. Source: Sandfire Dragon Ranch https://web.archive.org/web/20130422172203/http://www.sandfiredragonranch.com/index.php?main_page=product_info&products_id=80.
- Genus Echinotriton (2 species)
Spiny Newt kind

Fig. 40. Spiny newt kind. Source: Washington Department of Fish and Wildlife http://wdfw.wa.gov. Photo: Henk Wallays.
- Genus Euproctus (2 species)
Brook Newt kind

Fig. 41. Brook newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Ichthyosaura (1 species)
Alpine Newt kind

Fig. 42. Alpine newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Laotriton (1 species)
Laos Newt kind

Fig. 43. Laos newt kind. Source: Mongabay.com http://www.mongabay.com. Photo: Bryan Stuart.
- Genus Lissotriton (5 species)
Small-Bodied Newt kind

Fig. 44. Small bodied newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Neurergus (4 species)
Spotted Newt kind

Fig. 45. Spotted newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Notophthalmus (3 species)
Eastern Newt kind

Fig. 46. Eastern newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Ommatotriton (2 species)
Banded Newt kind

Fig. 47. Banded newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Pachytriton (8 species)
Paddle-Tail Newt kind

Fig. 48. Paddle-tail newt kind. Source: Washington Department of Fish and Wildlife http://wdfw.wa.gov. Photo: Henk Wallays.
- Genus Paramesotriton (11 species)
Warty Newt kind

Fig. 49. Warty newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Pleurodeles (3 species)
Ribbed Newt kind

Fig. 50. Ribbed newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Taricha (4 species)
Pacific Newt kind

Fig. 51. Pacific newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Triturus (7 species)
Crested Newt kind

Fig. 52. Crested newt kind. Source: Wikipedia http://en.wikipedia.org.
- Genus Tylototriton (14 species)
Crocodile Newt kind

Fig. 53. Crocodile newt kind. Source: Wikipedia http://en.wikipedia.org.
Family Proteidae
Family Proteidae contains two genera; Proteus (one species) and Necturus (five species) with an average total length of 25 cm. Fossils have been found from the Upper Paleocene in North America and to the Middle Miocene in Europe and Kazakhstan (Larson, Wake, and Devitt 2006; Petranka 1998, p. 417). Proteids are paedomorphs and even though adults have lungs, they are perennibranchiate and retain filamentous gills throughout their lives (Amphibiaweb 2013). Other shared characters include; maxillae and septomaxillae absent, a reduction in the number of toes, and a diploid number of 38 (Amphibiaweb 2013). Members include the Olm or the European blind salamander (Proteus anguinus) which is a troglobite that may be capable of a degree of viviparity (Amphibiaweb 2013) and has a cognitum that superficially fits with certain plethodontid salamanders. The other members are waterdogs and mud puppies (Necturus). While it is too difficult to determine from what kind they have diversified from, their obligate aquatic morphology and paedomorphy suggest a post-Flood phenomenon and I do not include them as an Ark kind.
Family Sirenidae
The sirens are long aquatic, eel-like salamanders with an average total length of 40 cm with small forelimbs, absent hind limbs, and no pelvic girdle (Amphibiaweb 2013). The family consists of two genera (Pseudobranchus/Siren) and four species that are found in the eastern United States and northeastern Mexico (Petranka 1998, p. 479). Fossil sirenids are known from the middle Eocene (Wyoming), middle Miocene (Nebraska and Texas), and the lower Miocene and Pleistocene (Florida) while Pseudobranchus fossils are known from Florida Pliocene and Pleistocene strata (Petranka 1998, p. 479). They are obligate neotenes, and like Proteidae, are perennibranchiate. Characters shared include no premaxillary or maxillary teeth. As with Proteidae, their obligate aquatic morphology and neoteny suggest that they are products of post-Flood diversification and therefore I don’t include them as an Ark kind.
Order Gymnophiona (The Caecilian Kind)
Probably the least familiar order of burrowing or aquatic Lissamphibians, Gymnophiona (clade Apoda), currently consists of 10 Families and 191 species with an average total length of 35 cm (Amphibiaweb 2013; Kamei et al. 2012; Pough et al. 2004, p. 61). Many live in moist soil and because of this fossorial existence, it is very difficult to study their life history. Common characters include long annulated (ringed) bodies, reduced or absent tails, absent limbs and girdles, reduced eyes covered by skin or bone, reduced or absent left lungs, well ossified skulls, and scales (unique to Lissamphibians) located in the dermis below the annular grooves and next to the poison glands (Pough et al. 2004, pp. 57–60). Their annulated body is characteristic caecilian morphology and each annulus is associated with a rib (Pough et al. 2004, p. 58). They also have a unique structure called a tentacle that is located between the eyes and nostrils in a little chamber that opens at the skull surface. It is a chemosensory organ that is positioned differently depending on the species and is helpful for species identification (Pough et al. 2004, p. 58).
They also have a unique dual jaw adductor mechanism not found in other tetrapods. This mechanism consists of two muscles; the mandibular adductors (found on ancestral tetrapods) and interhyoideus muscles (unique to caecilians) that are working together (Pough et al. 2004, p. 58).
They internally fertilize and males have a copulatory organ called a phallodeum that can transfer sperm to a female. About 70% of the females are oviparous and eggs are laid in terrestrial or aquatic sites (Pough et al. 2004, p. 58). If eggs are laid on land, most are laid in strings and the female protects them. About 30% of the caecilians are viviparous and egg yolk volumes are much reduced compared to oviparous eggs because nutrients are supplied by the mothers from special cells in her oviduct (Pough et al. 2004, p. 58). Larvae have lungs, gill slits, lateral line systems, and caudal fins until they metamorphose to adulthood. Like salamanders, metamorphosis is gradual and lungs may remain (with some lungless exceptions), the tentacle develops, caudal fins and lateral lines disappear, and gill slits close (Pough et al. 2004, p. 60).
Fossil caecilians are known from the early Jurassic of Arizona, lower cretaceous of Morocco, late Cretaceous of Bolivia and Sudan, and from the Paleocene to the Pleistocene in Brazil and Bolivia (Pough et al. 2004, p. 60). The best preserved fossils, like Eocaecilia micropodia, come from the Jurassic and share many derived characters with extant caecilians. They differ in that E. micropodia have well developed limbs and girdles, though they are reduced (Pough et al. 2004, pp. 58, 60). A brief description of each family follows. (See also table 2).
Family Caeciliidae
Caeciliidae consists of two genera and 42 species whose range is South and Central America and average total length is unknown. However, there are very large specimens between 60–100 cm (Amphibiaweb 2013). Shared characters include imperforate stapes (ear bones), inner mandibular teeth and eyes surrounded or covered by the maxillopalitine (upper jaw bone) regions of the skull (Amphibiaweb 2013).
Family Chikilidae
This newly described family has only one genus and one species and little is known about them. They are very similar to other caecilians and have been found in India (Amphibiaweb 2013; Kamei et al. 2012).
Family Dermophiidae
Consisting of four genera and 14 species, they have an average total length of 50 cm. Dermophiids have secondary annuli (subdivisions of primary annuli) and are found in Africa, Central America, and South America (Amphibiaweb 2013).
Family Herpelidae
Herpelidae contains two genera and nine species and shared characters include perforate stapes, multiple small antotic foramina (skull openings), and both prefrontals and septomaxillae are separate. They are found in Africa and have an average total length of 33 cm.
| Description | Caeciliidae | New Family Chikilidee |
Dermophiidae | Herpelidae | Ichthyophiidae | Indotyphlidae | Rhinatrematidae | Scolecomorphidae | Siphonopidae | Typhlonectidae |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of genera | 2 | 1 | 4 | 2 | 3 | 7 | 2 | 2 | 7 | 5 |
| Number of species | 42 | 1 | 14 | 9 | 52 | 21 | 11 | 6 | 22 | 13 |
| Average Total Length (cm) | unknown 60-100 +/- | unknown | 50 | 33 | 32 | 22 | 25 | 36 | contains smallest: 11 | contain largest: avg. 45 |
| stapes (ear bones) | imperforate | unknown | present | perforate | present | imperforate | present | none | imperforate | present |
| embryo development | ovi or viviparous | oviparous | ovi or viviparous | oviparous | oviparous | ovi or viviparous | oviparous | ovi or viviparous | oviparous | viviparous |
| annuli | primary/some secondary | primary | secondary | primary/some secondary | secondary/tertiary | primary | secondary/tertiary | primary | primary/some secondary | primary |
| dual jaw adductor mechanism | 2 muscle bundles | 2 muscle bundles | 2 muscle bundles | 2 muscle bundles | 2 muscle bundles | 2 muscle bundles | *1 muscle bundle* | 2 muscle bundles | 2 muscle bundles | 2 muscle bundles |
| dermal scales | present | present | present | present | present | absent | numerous | absent | none | none |
| aquatic or semiaquatic | no | no | no | no | no | no | no | no | no | YES |
| intrauterine feeding by fetus | no | unknown | no | no | no | depends on species | no | depends on species | no | YES |
| juveniles shed gills early | no | no | no | no | no | no | no | no | no | YES |
Family Ichthyophiidae
Ichthyophiidae contains three genera and 52 species with an average total length of 32 cm. They have true tails, a counter sunk lower jaw, and an advanced dual jaw closing mechanism. Their range occurs in south and southeast Asia (Amphibiaweb 2013).
Family Indotyphlidae
This family has seven genera and 21 species with an average total length of 22 cm. Characters shared include; imperfect stapes, inner mandibular teeth, bicuspid teeth, an eye located at the border of the squamosal (a skull bone) and maxillopallatines, and lacks scales and secondary annuli (Amphibiaweb 2013). Some are viviparous and others are oviparous and they are found in Africa, India, and the Seychelles.
Family Rhinatrematidae
The two genera and 11 species in Rhinatrematidae are all oviparous and are found in northern South America through Brazil, Columbia, Ecuador, Peru, Surinam, Guyana, French Guiana, and Venezuela (Amphibiaweb 2013). The average total length is 25 cm and they share the following characters; true short tails with post-cloacal vertebrae, mouth is terminal rather than counter sunk, tentacular opening is adjacent to the eye rather than anterior as found in other caecilians, and primary annuli are subdivided into secondary and tertiary annuli (Amphibiaweb 2013). As was described above, all caecilians have a unique dual jaw adducting mechanism. The difference with Rhinatrematidae is that they have one bundle of muscles where all the other caecilian families (clades) have two bundles (Amphibiaweb 2013).
Family Scolecomorphidae
Consisting of two genera and six species, they have an average total length of 36 cm and are native to western and eastern equatorial Africa (Amphibiaweb 2013). Shared characters include; countersunk lower jaws, tentacular openings far anterior on the snout, orbits are absent, eyes connected at the base of the tentacle and protrude when tentacle protrudes, lack stapes, annular grooves lack dermal scales, secondary and tertiary annuli are absent, and females have more vertebrae than males (Amphibiaweb 2013).
Family Siphonopidae
With seven genera and 22 species, Siphonopidae live in South America and are oviparous, have imperforate stapes, and lack inner mandibular teeth (Amphibiaweb 2013).
Family Typhlonectidae
Family Typhlonectidae have an average total length of 45 cm and currently contains five genera and 13 species. This group is aquatic or semi-aquatic and viviparous. Juveniles shed gills at an early stage, all make burrows at the water level or underwater, fetuses feed intrauterinely (extraordinary among Lissamphibians), possess tracheal lungs, have narial plugs, and lack annular scales and secondary annuli (Amphibiaweb 2013). One member of this family is known from only two specimens and is the largest lungless tetrapod (Atretochoana eiselti) known measuring 72.5 cm (Amphibiaweb 2013). They are found in northern South America and some consider them nested within Caeciliidae (Amphibiaweb 2013).
No hybridization data were found, but because of their unique characteristics, common terrestrial adaptations, and very strong cognita, I place the caecilian kind at the level of the Order and include them on the Ark.
Summary and Conclusions
After looking at the most current Lissamphibian systematics and recognizing that this data is in constant flux, I have made an initial estimate as to the number and identification of kinds from Orders Caudata and Gymnophiona that may have been represented on the Ark. Of the ten extant Caudate families, with the exception of Proteidae and Sirenidae because of probable post-Flood phenomena of their perennibranchiate morphology and obligate neoteny, the data suggests Noah had 53 Caudate kinds and one extant Gymnophionan kind represented on the Ark. Because of the strong cognita, many common and unique characters, and mostly terrestrial habits found in Caecilians I place the kind at the level of the Order and call them the Caecilian kind. These conclusions are tentative. It is my contention that with a proper biblical worldview, it is probable that future genetic research will bring us to a better understanding of what the data mean and how to interpret them in the light of biosystematics and taxonomy. No matter what the number turns out to be, there is no question that the Creator’s wisdom and desire for creatures to persist is a reflection of his marvelous diversity, loving provision, and promised salvation.
References
AmphibiaWeb. 2013. Retrieved from http://amphibiaweb.org/ on 5 January 2013.
Austin, S., J. Baumgardner, D. R. Humphreys, A. A. Snelling, L. Vardiman, and K. P. Wise. 1994. Catastrophic plate tectonics: A global flood model of earth history. In Proceedings of the third international conference on creationism, ed. R. E. Walsh, pp. 609–621. Pittsburgh, Pennsylvania: Creation Science Fellowship. Retrieved from http://www.answersingenesis.org/articles/aid/v5/n1/catastrophic-plate-tectonics on 6 September 2012.
Blackburn, D. C. and D. B. Wake. 2011. Class amphibia gray, 1825. In Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness, ed. Z.-Q. Zhang. Zootaxa 3148:1–237.
Brophy, T. R. and P. A. Kramer. 2007. Preliminary results from a baraminological analysis of the mole salamanders (Caudata: Ambystomatidae). Occasional papers of the BSG 10:10–24.
Dubois, A. 2005. Amphibia Mundi. 1.1. An ergotaxonomy of recent amphibians. Alytes 23 no. 1-2:1–24.
Duellman, W. E. and L. Trueb. 1986. Biology of amphibians. New York, New York: McGraw-Hill.
Duellman, W. E. ed. 1999. Patterns of distribution of amphibians: A global perspective. Baltimore, Maryland: Johns Hopkins University Press.
Fontenot, C. L. Jr. 2010. Amphiuma means (two-toed amphiuma) × A. tridactylum (three-toed amphiuma) hybridization. Herpetological Review 41:328.
Frost, D. R. (ed). 1985. Amphibian species of the world: A taxonomic and geographical reference. Lawrence, Kansas: Allen Press and Association of Systematics Collections.
Frost, D. R. et al. 2006. The amphibian tree of life. Bulletin American Museum of Natural History 297:1–370.
Good, D. A. and D. B. Wake. 1992. Geographic variation and speciation in the torrent salamanders of the genus Rhyacotriton (Caudata: Rhyacotritonidae). Zoology vol. 126. Berkley, California: University of California Press.
Hennigan, T. 2010. Reproductive thievery in salamanders? Answers in Depth. Retrieved from http://www.answersingenesis.org/articles/aid/v5/n1/reproductive-thievery-in-salamanders on 23 August 2012.
Heying, H. 2003. Animal Diversity Web. Retrieved from http://animaldiversity.ummz.umich.edu/accounts on 13 September 2012.
International Union for Conservation of Nature and Natural Resources. 2012. Retrieved from http://www.iucnredlist.org/amphibians on 27 August 2012
Jensen, J. B., C. D. Camp, J. W. Gibbons, and M. J. Elliott (ed). 2008. Amphibians and reptiles of Georgia. Athens, Georgia: University of Georgia Press.
Kamei, R. G., D. S. Mauro, D. J. Gower, I. V. Bocxlaer, E. Sherratt, A. Thomas, S. Babu, F. Bossuyt, M. Wilkinson, and S. D. Biju. 2012. Discovery of a new family of amphibians from northeast India with ancient links to Africa. Proceedings of the Royal Society of Biological Sciences: 1–7. Retrieved from http://rspb.royalsocietypublishing.org/content/early/2012/02/15/rspb.2012.0150.full on 18 September 2012.
Kragl, M., D. Knapp, E. Nacu, S. Khattak, M. Maden, H. H. Epperlein, and E. M. Tanaka. 2009. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460:60–65.
Larson, A., D. Wake, and T. Devitt. 2006. Caudata. Salamanders. Retrieved from http://tolweb.org/Caudata/14939/2006.09.05 on 1 September 2012.
Lightner, J. K., T. Hennigan, G. Purdom, and B. Hodge. 2011. Determining the Ark kinds. Answers Research Journal 4: 195–201. Retrieved from http://www.answersingenesis.org/articles/arj/v4/n1/ark-kinds-flood-baraminology-cognitum.
Litvinchuk, S. N., L. J. Borkin, and J. M. Rosenov. 2004. Intraspecific and interspecific genome size variation in hynobiid salamanders of Russia and Kazakhstan: Determination by flow cytometry. Asiatic Herpetological Research 10:282–294.
McNeil, R. 2010. A monster opportunity. Conservation International. Retrieved from http://www.conservation.org/FMG/Articles/Pages/monster_opportunity_japan_salamander.aspx on 5 September 2012.
Page, R. B, J. R. Monaghan, and J. A. Walker. 2009. A model of transcriptional and morphological changes during thyroid hormone-induced metamorphosis of the axolotl. General and Comparative Endocrinology 162, no. 2:219–232.
Petranka, J. W. 1998. Salamanders of the United States and Canada. Washington D.C.: Smithsonian Institution.
Pough, F. H., R. M. Andrews, J. E. Cadle, M. L. Crump, A. H. Savitsky, and K. D. Wells. 2004. Herpetology, 3rd ed. Upper Saddle River, New Jersey: Pearson Prentice Hall.
Pyron, A. and J. J. Wiens. 2011. A large-scale phylogeny of Amphibia with over 2,800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution 61, no. 2:543–583.
Sanders, R. W. and K. P. Wise. 2003. The cognitum: A perception-dependent concept needed in baraminology. In Proceedings of the fifth international conference on creationism, ed. R. L. Ivey, pp. 445–456. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Snelling, A. A. 2009. Earth’s catastrophic past: Geology, Creation and the Flood, 2 vols. Santee, California: Institute for Creation Research.
Tree of Life. n.d. Retrieved from http://tolweb.org/tree/phylogeny.html on 13 August 2012.
Turner, K. J. 2009. The kind-ness of God: A theological reflection of mîn, “kind.” In CORE Issues in Creation, no. 5, ed. T. C. Wood and P. A. Garner, pp. 31–64. Eugene, Oregon: Wipf and Stock.
Vasilyan, D., M. Böhme, and M. Winklhofer. 2010. The palaeoclimatic significance of Eurasian giant salamanders (Cryptobranchidae: Zaissanurus, Andrias)—indications for elevated humidity in central Asia during global warm periods (Eocene, late Oligocene warming, Miocene Climate Optimum). Geophysical Research Abstracts, vol. 12. Retrieved from http://www.wahrestaerke.com/~madelaine/EGU2010_Andrias.pdf on 4 September 2012.
Voss, S. R. and J. J. Smith. 2005. Evolution of salamander life cycles: A major-effect quantitative trait locus contributes to discrete and continuous variation for metamorphic timing. Genetics 170, no. 1:275–281.
Williams, P. J. 1997. What does min mean? CEN Tech J. 11 no. 3:344–352.
Wood, T. C. 2003. Mediated design. Impact Article #363. Institute for Creation Research: El Cajon, California. Retrieved from http://www.icr.org/i/pdf/imp/imp-363.pdf on 28 June 2012.
Wood, T. C. 2006a. The current status of baraminology. Creation Research Society Quarterly 43, no. 3:149–158.
Wood, T. C. 2006b. Statistical baraminology workbook. Unpublished workbook presented at workshop during the BSG conference June 13, 2007, Liberty University, Lynchburg, Virginia.
Woodmorappe, J. 1996. Noah’s Ark: a feasibility study. Santee, California: Institute for Creation Research.