The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
An unbridgeable gap exists between the simple urinary system used in invertebrates and the far more complex kidney system used in all vertebrates. No direct evidence of the evolution of one system into the other exists, nor have any viable “just-so” stories been proposed to explain the evolution of the simple invertebrate urinary system into the complex vertebrate kidney-urinary system. The common reason evolutionists give for the lack of evidence to bridge this chasm is that soft tissue is usually not preserved in the fossil record.
The problem with this claim is thousands of so-called “living fossils” exist that are claimed to be anatomically close to their hundreds of millions years old design which should display evidence of the less evolved organs. Thus, if kidney evolution occurred, evidence of primitive kidneys in living animals that bridged the two very different systems would exist. Comparisons with living fossils reveals a “paleontologic record [that] is ambiguous and open to controversy” (Romer and Parsons 1986, 399).
Another problem for evolution is that a design is employed even in the simplest, least-evolved mammals, that is very similar to that used in the highest-evolved primates, including humans (Romagnani, Lasagni, and Remuzzi 2013). All vertebrates use very close to the same design, and the few variations that exist are relatively minor. The main reason the designs are very similar is that, functioning effectively to remove waste products requires a specific, irreducibly complex kidney design.
Keywords: kidney anatomy and physiology, evolution of body organs, fossil record, intelligent design, irreducible complexity, living fossils
Introduction
Mahasen opines that, the “Evolution of the kidney is a hot topic for many researchers and biologists as there is no better place to see the impact of evolutionary pressures on organ development than in the kidney” (Mahasen 2016). This review examines the evidence for this “hot topic,” of kidney evolution from the simple system that functions to remove waste products in invertebrates. The fact is, “the human kidney appears to be extraordinarily complex” and well designed (Vize 2004, 344). The kidney is also
an excellent biochemical model showing design in nature. Design implies a designer. The development of the kidney follows a very precise pattern and time schedule. The anatomy and physiology of the kidney and the entire urinary system are complex and precise. . . The kidneys perform an incredible function. In one day’s time, they filter the equivalence of sixty times the total plasma. (Speck 1994, 505, 508).
The Importance of the Kidney
The kidneys’ critical importance, as summarized by Vize, is: “the composition of the blood is determined not by what the mouth ingests but by what the kidneys keep; they are the master chemists of our internal environment” (Vize 2004, 344). They also “remove from the blood the infinite variety of foreign substances which are constantly being absorbed from our indiscriminate gastrointestinal tracts,” (Vize 2004, 345) a function which is actually part of the critical
task of keeping our internal environment in an ideal, balanced state. Our glands, our muscles, our bones, our tendons, even our brains, are called upon to do only one kind of physiological work, while our kidneys are called upon to perform an innumerable variety of operations. Bones can break, muscles can atrophy, glands can loaf, even the brain can go to sleep, without immediately endangering our survival, but when the kidneys fail to manufacture the proper . . . blood [composition] neither bone, muscle, gland nor brain can carry on (Vize 2004, 344; emphasis added).
The primary material the mammalian kidney removes is urea, the major waste product of protein metabolism, but numerous toxins are also removed. The kidneys must produce a healthy balance in the blood of water, salts, and minerals (including especially sodium, calcium, phosphorus, and potassium). The kidneys also remove waste products produced by the body’s somatic cells (Ringoir, Vanholder, and Massry 2012).
Basic Kidney Types and Functions
The invertebrate excretory-kidney system is generally divided into three basic types—contractile vacuoles, protonephridia, and the true kidney, the metanephridium.
The contractile vacuoles used in protozoa, such as the amoeba, are not true excretory organs but function primarily to remove water to regulate the organism’s electrolyte balance. Excess water is collected in vesicles surrounding the cell membrane, which are then emptied into contractile vacuoles. When the contractile vacuoles accumulate enough fluid, the excess passes through a membrane pore into the surrounding environment. The cellular wastes are also excreted by ordinary exocytosis by contractile vacuoles merging with the cell membrane, then expelling the wastes into the environment.
Some fish have chloride-secreting glands on their gills, while iguanas, marine turtles, snakes, and birds that scoop fish out of the water, all have salt glands that excrete excess salt. Thus they serve some of the same functions as kidneys (Kent 1992, 510). These salt glands, however, use a very different design than kidneys and, therefore, are not considered by evolutionists to be evolutionary precursors to kidneys. Furthermore, all of these creatures have kidneys in addition to salt glands, so one would not expect the salt gland to have evolved into the kidney or vice versa. Rather, they are extra-renal salt-excretion mechanisms that are not located in the same place as kidneys (Kent 1992, 510). For example, the salt excretion glands of lizards (for example, the chuckwalla) and some marine birds are located near the eye orbits and empty into the nasal canals (Kent 1992, 510–511). Sweat glands are another extra-renal method that mammals use to eliminate excess salt.
The protonephridia design consists of flame cells. Flame cells are a cluster of cilia that resemble a flickering flame when microscopely examined (Valverde-Islas et at. 2011). The cilia propel waste matter through the tubules and out of the body through excretory pores on the body surface. This system is use in flatworms, annelids, nemertines and rotifers.
The First True Kidney
Metanephridium is the first system that could be properly called a kidney (see figs. 1 and 2). It is used in many invertebrate animals including crustaceans, arthropods, mollusks, and annelids. This system consists of a series of nephridium tubules that terminates into a ciliated flame cell where specific ions and molecules are reabsorbed. Materials remaining in the flame cells, including water, surplus ions, metabolic waste, toxins, and excess hormones, are removed from the organism by directing them down funnel-shaped ciliated bodies called nephrostomes. These wastes are then expelled into the environment through an excretory pore called the nephridiopore, located on the animal’s body surface (Buchsbaum 1987, 299).
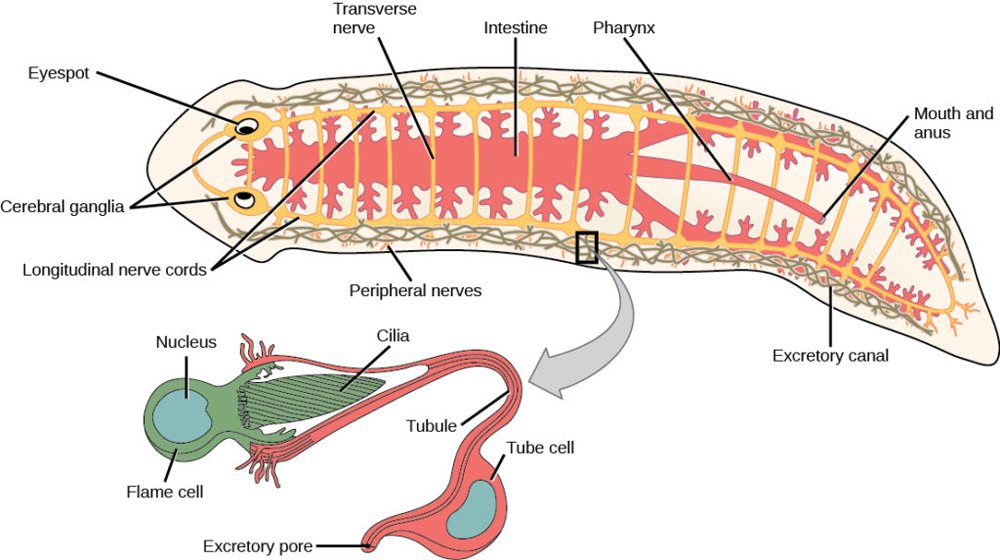
Fig. 1. Metanephridium in a planarian flatworm. Contrast this with the vertebrate kidney in fig. 2. Image: https://courses.lumenlearning.com/suny-wmopen-biology2/chapter/superphylum-lophotrochozoa/.
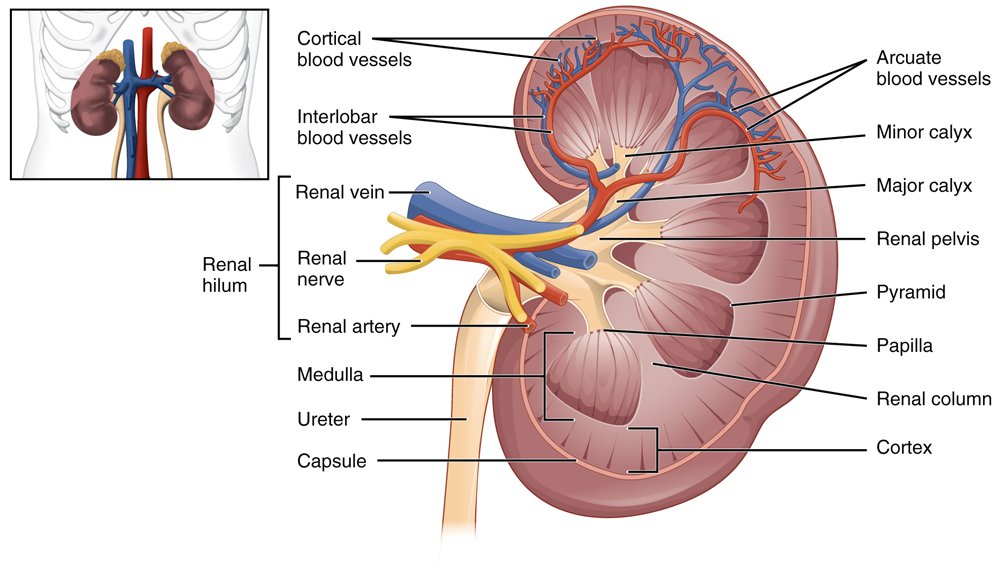
Fig. 2. A cross section of the body of the human kidney. Image: https://upload.wikimedia.org/wikipedia/commons/8/87/2610_The_Kidney.jpg. OpenStax College, CC BY 3.0 <https://creativecommons.org/licenses/by/3.0>, via Wikimedia Commons.
Malpighian tubule is a comparatively simple osmoregulatory system used in insects, myriapods (including millipedes and centipedes), arachnids, and tardigrades (that is, water bears). The system consists of thin, delicate, convoluted, blind-branching tubules extending away from the alimentary canal. The excretory system is formed by a bank of Malpighian tubules. Malpighian tubules are slender tubes a single cell thick in the posterior regions of arthropods. The distal end is closed off and the proximal end joins the alimentary canal at the junction between the midgut and hindgut (Jordan and Verma 2001, 906). Most of the normally highly-convoluted tubules absorb solutes, water, and wastes from the surrounding hemolymph. They contain thin actin fibers for structural support and microvilli with cilia to move substances within the tubules (Bradley 1985).
Body fluids are drawn into the tubules by osmosis and then most of the fluids are reabsorbed. Nitrogeno us and other wastes that are not reabsorbed are emptied into the insect’s gut. This excretory system effectively conserves water and is especially suitable for insects inhabiting dry environments (Hickman, Roberts, and Larson 2001, 670). In contrast to vertebrates, this system lacks a blood supply.
The Vertebrate Kidney
All vertebrates utilize kidneys that are enormously more complex than the systems noted above. Furthermore, all vertebrate kidneys are very similar in design to the human kidney. However, minor “differences in the structure and function of various vertebrate kidneys” exist to better adapt them to the specific environment that the animal lives (Mahasen 2016).
An example of a slightly different vertebrate kidney design is that used in the kangaroo rat. Kangaroo rats require a highly efficient water retention system because they subsist in a very dry area. Therefore, kangaroo rats must achieve an extremely efficient water intake/loss ratio to survive. The minor modification to achieve this goal includes a longer water retention structure called the ‘loop of Henle’ the structure designed to retain water (see figs. 3 and 4). Water retention is proportional to the loop-of-Henle length. The longer its length, the greater its absorption (Munkácsi and Palkovits 1965, 303).
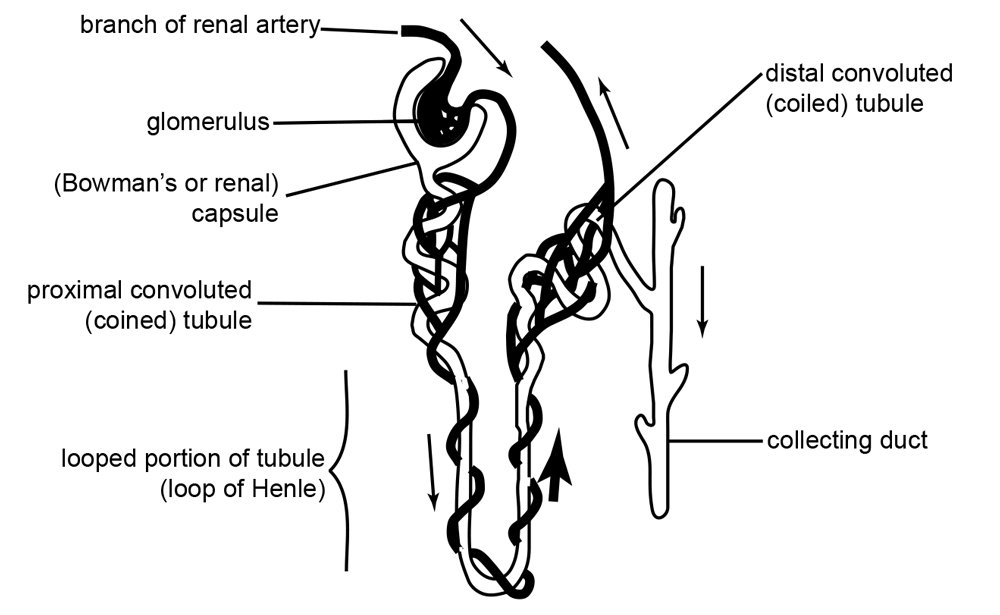
Fig. 3. Diagram of a nephron. Each human kidney contains millions of nephrons. https://upload.wikimedia.org/wikipedia/commons/1/16/Anatomy_and_physiology_of_animals_Kidney_tubule_or_nephron.jpg. The original uploader was Sunshineconnelly at English Wikibooks., CC BY 3.0 <https://creativecommons.org/licenses/by/3.0>, via Wikimedia Commons. English: by Ruth Lawson, Otago Polytechnic.
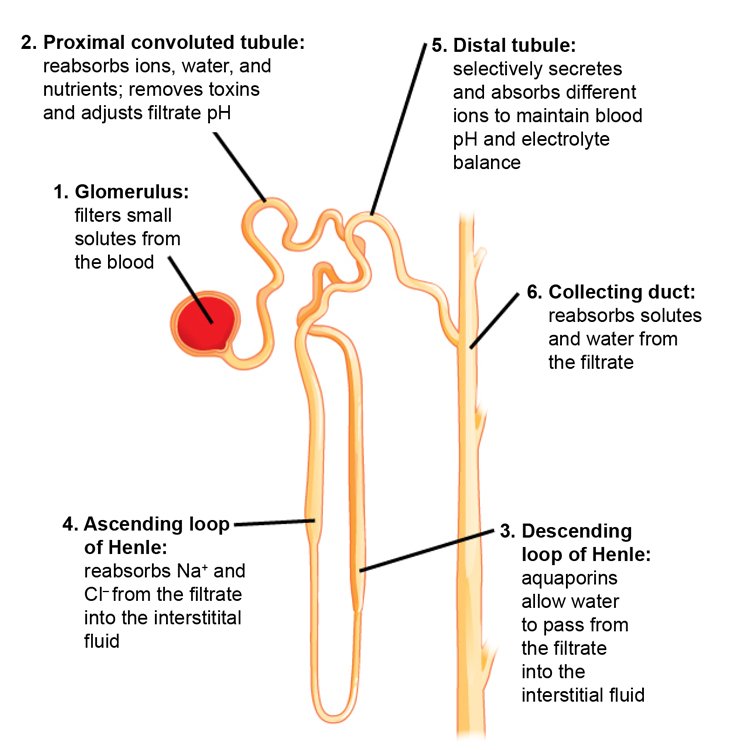
Fig. 4. The nephron is the functional unit of the kidney. The diagram shows the basic function of each part. From Wiki Commons. https://opentextbc.ca/biology/wp-content/uploads/sites/96/2015/03/Figure_41_03_04.jpg.
Of the normal variations for the loop-of-Henle length, the longer variation would logically be favored in these animals. While selection can explain the difference existing in kangaroo rats compared with other mammals, it cannot explain the evolution of the vertebrate kidney itself.
Kidney Recapitulation Theory
The radically new design required to produce a kidney from scratch cannot function until all of the necessary parts exist and are functioning. Until that time toxic waste would accumulate and as a result vertebrate life forms could not survive. This is shown by the fact that several different structures are a required part of embryonic development to excrete wastes during its entire development path. Early in embryonic development, a structure similar to the metanephridium exists, then next, one similar to the Malpighian tubule system develops. One of the most cited examples of recapitulation for decades was this development of the mammalian kidney (Davidheiser 1969, 244–245). Only when all of the required part for a functional kidney are in place can the kidney function. Until then the embryo uses the temporary structures described above.
The Kidney Recapitulation Theory is the claim that the kidney’s progression in embryo development is an example of ‘ontogeny recapitulates phylogeny.’ In other words, as the embryo develops it supposedly repeats the evolution of the kidney from the primitive nephridia to the vertebrate kidney. Professor Arey claims, “Nowhere can a better illustration of the principle of Recapitulation” be found (Arey 1942, 261). The first kidney development stage in vertebrates is the pronephros which resembles the kidney of the most primitive type of fishes, the jawless fish (that is, the lamprey and hagfish). The pronephros is necessary for the development of other structures that remain in the body after the pronephros is replaced (Frair and Davis 1983, 43). The next stage is the mesonephros which resembles that of the jawed fishes and amphibians. The last stage, the metanephros, is the adult kidney used in all terrestrial reptiles, birds and mammals.
As evidence for the recapitulation theory, it is noted that, like human embryos, many invertebrates excrete nitrogen compounds such as ammonia from the breakdown of protein. One problem with applying kidney recapitulation to biological evolution is ammonia and urea (and presumably uric acid) are not products of the kidney, but rather of the liver. The kidney excretes what the liver produces from protein metabolism and breakdown. Without the kidney, the liver will poison the organism by the toxins it produces. Evidence of this development is embryonic birds excrete ammonia, then, as they develop their kidney, excrete urea, and adult birds all excrete uric acid (Klotz 1970, 150).
Kidney Recapitulation Theory Refuted
In the cases cited above, in humans the stages are actually not progressive steps to an adult kidney, but are three different fully-functional excretory organs, and only the last stage is retained into adulthood (Davis et al. 1989, 129). Another major problem with this theory is that some embryos of very closely allied species follow very different embryonic paths, not only of kidney development, but of other organ development as well. This kidney recapitulation claim was in the past seen as one of, if not the best, example of recapitulation, but for good reasons is rarely cited in evolutionary textbooks today (Shute 1971, 40–41). Also, many kidney traits are not consistent with mammal evolution.
For example, the human kidney is divided into separate pyramid shaped structures called appropriately pyramids (see fig. 2 again) which contain between 9 and 20 renal papillae (collecting duct openings). Each pyramid typically contains one renal papilla. The papilla is the apex of the pyramid where several converging collecting ducts open into a minor calyx.
The mouse, gerbil, rat, guinea pig, rabbit, dog, and cat kidneys have only a single lobe in contrast to the multi-lobed kidney shared with, for example, monkeys, cattle, and pigs (Bach, Bridges, and Mudge 1985, 218). The reniculate kidney is a multilobed design used in marine and aquatic mammals such as pinnipeds (seals, sea lions, and walruses) and cetaceans (dolphins and whales) but absent in terrestrial mammals except bears.
Needham notes that, instead of recapitulation, the progression reflects increasing levels of complexity as development occurs so as to be able to process waste products more completely, thereby improving waste removal more efficiency and, thereby, reducing toxicity levels (Needham 1930, 154). Another problem with the recapitulation conclusion is that, although some resemblances to the theoretical evolutionary progression exist, many clear differences also exist. The human embryo must, even while developing, remove waste products at all stages of development, a function carried out in human fetuses by the placenta. The design in the early stages of development uses these simple systems to survive until replaced by the vertebrate kidney, but only when it is functional.
A significant problem with recapitulation theory is that, as the embryo increases in size, the new kidney does not develop from the old one but is formed behind the first one. Only when it is fully developed does the new kidney replace the earlier kidney. The first kidney then atrophies. This process solves an important anatomical requirement. The embryonic condition requires the kidney to be situated far forward anteriorly, but as development proceeds this position is very problematic for the adult, so the new kidney is located posteriorly to the earlier kidney (Dewar 1957, 198). The problem of having both of your kidneys in the front of your abdominal cavity creates the problem of having this essential organ in the relatively less protected anterior abdomen rather than its current well-protected by strong back muscles in the posterior adult location.
In response to the claim that “renal repair recapitulate kidney development?” one research study concluded: “Although it is tempting to suggest that the response of the kidney to injury is to reinitiate developmental processes, the cell types present in the postnatal kidney are completely different . . . [from] those evident during development” (Little and Kairath 2017, 42).
Another problem applying kidney recapitulation theory to biological evolution is survival is achieved because the mother’s kidneys allow effective waste removal during the entire time of embryonic and fetal kidney development. The last problem is no specific evidence exists for kidney evolution, thus the parallel is interesting, but does not add support to the theory. As the late Harvard Professor Alfred Romer concluded, it is often claimed that in human development exist three “distinct kidneys which have succeeded one another phylogenetically as they do embryologically . . . there is little reason to believe this. The differences are readily explainable on functional grounds” (Romer and Parsons 1986, 407). The stages are separate, develop successively, and are designed to function only at a specific time in development (Clark 1967, 28).
Evolution of the Vertebrate Kidney from the Invertebrate Kidney
The focus here is on the evolution of the kidney from the hypothetical invertebrate design called the proto-vertebrate kidney system to the vertebrate kidney seen in vertebrates today. The proto-vertebrate term is used because it is believed that the invertebrate excretory system evolved into the very different vertebrate designs existing today from one very early proto-kidney. As noted above, the kidney design is basically the same in all adult vertebrates, and the few differences are minor: “Variations in details from fish to humans are principally in the number and arrangement of glomeruli and in the relative complexity of the tubules” (Kent 1992, 495). Examples of minor differences include the body design of snakes, lizards, and a group of caecilians (limbless amphibians) called apodans. These animals require an elongated kidney design, which is what exists in these animals (Kent 1992, 510). A single kidney lobe contains many kidney lobules featuring a lobulated design, where each cluster contains many tubules, a design used in reptiles, birds, and even mammals.
The Human Kidney Design and Function
The human kidney is a bean-shaped structure about the size of a human fist. Aside from the central nervous system and the liver, it is one of the most complex organs in the human body. One primary function is to remove metabolic wastes from the blood using the approximately one million nephrons called uriniferous tubules (Smith 1953, 139). The total filtering area of these nephrons shown in figs. 3 and 4 is larger than the size of a singles tennis court 78 ft by 27 ft (that is, over 2,106 ft2) which fits into an area the size of your fist! The kidneys filter from 120 to 152 qt (113 to 144 l) of blood at the rate of 1200 cc per minute to create 1 to 2 qt (0.94 to 1.8 l) of urine daily. This rate equals 1700 l (1800 qt) of blood filtered per day (Smith 1953, 139). Blood flow is often a relative indicator of the importance of an organ. The kidneys receive one-fourth of the body’s blood supply every minute, far outdistancing any other organ including the brain!
In a very simplified outline of the kidney’s function, the kidneys receive blood from the paired renal arteries. Each renal artery then branches into segmental arteries which divide further into interlobar arteries. The interlobar arteries become arcuate arteries, which become interlobular arteries which give rise to afferent arterioles that penetrate the glomerulus tuft of capillaries which filters the blood. Lastly, the filtrate is collected into a cup-like sac named the Bowman’s capsule.
The glomerulus allows proteins less than 30 kilodaltons (a weight of 30,000 hydrogen atoms) and small molecules (water, glucose, salt (NaCl), amino acids, and urea) to pass freely into the Bowman’s space, resulting in a filtrate similar to blood plasma (see figs. 3 and 4). The filtrate, called the ultrafiltrate, consists of whole blood minus blood cells, platelets, and large proteins which cannot pass through the glomerulus. These units are returned to the circulating blood.
The ultrafiltrate is then transferred into the proximal convoluted tubule (connected to the Bowman’s capsule), then through the loop of Henle, (including a sharp bend in the renal medulla going from the loop of Henle’s thin descending limb to its thin-to-thicker ascending limb), and finally to the distal convoluted tubule. Water, glucose, amino acids, and the electrolytes such as sodium, chloride, potassium, and calcium are reabsorbed in the proximal convoluted tubule while urea, uric acid, creatinine, sodium, potassium, and hydrogen ions are secreted into the filtrate in the distal convoluted tubule (see fig. 2 again). Each section of the tube returns elements back into blood circulation according to the body’s needs at the time.
The thin ascending limb close to the “loop tip” of the loop of Henle is impermeable to water, but is permeable to sodium (Na+), potassium (K+) and chloride (Cl−) ions which are reabsorbed from the urine by active transport using a Na-K-Cl co-transporter. The distal convoluted tubule located in the kidney’s cortex reabsorbs calcium, sodium, and chloride and regulates urine pH by secreting protons and absorbing bicarbonate. After the filtrate remaining is processed in the tubules, urine is formed and transported via the collecting duct to the (urinary) bladder for disposal.
The kidney is interconnected with the body in many ways, especially hormonally. One example is the fluid regulation system which controls water reabsorption. The body’s fluid needs are determined by the nine-amino-acid hormone vasopressin, an anti-diuretic, (that is, a substance that suppresses the formation of urine, thus retaining water). After the sensor system determines the body’s water needs, if a dehydration condition is detected, a message is sent to the pituitary gland to manufacture vasopressin, which is then sent into the bloodstream.
The vasopressin binds onto the vasopressin receptor on the kidney tubule cell, causing increased water reabsorption. If the water fluid is sufficient, the hypothalamus will reduce the level of vasopressin produced by the pituitary sent to the kidney, causing it to reabsorb less water, consequently increasing the volume of water in the urine. The half-life of vasopressin is only a minute or two, allowing for fine monitoring and control of body-fluid levels. Loss of fluid control is lethal in a matter of hours, thus this system is critical for life, as is the kidney itself. This is only one regulation system that is part of several even more complex systems that interface with the kidney.
Some of the kidney’s other critical life functions include maintaining overall body fluid balance, regulating minerals in the blood, filtering waste materials from food, and removing medications and toxic substances from the blood. It also produces hormones that help to regulate red blood cell production, and blood pressure and promote bone health (Brewstera and Perazella 2004). It even helps to regulate the chemical composition of body fluids.
In order to function, the kidney requires a sufficient blood pressure level. Consequently, long-term regulation of arterial blood pressure is controlled principally by the kidneys. Special purinoceptors are broadly distributed in renal tubular and vascular structures to achieve segmental control of renal vascular resistance, auto-regulation, tubular re-absorptions, and a mechanism for regulating extracellular fluid volume and, ultimately, blood pressure (Beusecum and Inscho 2015, 82). Another kidney blood-pressure control mechanism is the renin-angiotensin-aldosterone system which regulates renal vasomotor activity, maintains optimal salt and water homeostasis, and regulates kidney tissue growth (Brewstera and Perazella 2004, 263).
The Lack of Evidence for the pre-Kidney in the Fossil Record
No clear evidence exists for pre-kidney phylogeny. Even basic questions are unanswered, such as where this pre-kidney evolution occurred. For example, the question of whether “the first vertebrates, hence vertebrate kidneys, evolved in fresh or salt water is not settled . . . Stahl presents both sides of the debate, pointing out that negative evidence—in this instance, absence of fossil evidence—is not conclusive” evidence (Kent 1992, 495; emphasis added).
Evolutionary Theory Proposals
One of the most important theories of kidney evolution was published in 1953 by Homer W. Smith, a kidney specialist, whose ideas were reprinted in 2004 (Vize 2004). Smith’s theory is creative speculation void of any empirical evidence. This is why he calls his theory the philosophy of kidney evolution, not the science of kidney evolution (Smith 1953). In short, his theory postulates that “selection led to adaptation of the kidney in response to the move [of life] from salt—to freshwater, and . . . back to saltwater again” (Vize 2004, 344).
His theory was constructed from his understanding that regulation of body fluids is the main function that all kidney systems have in common. His theory also illustrates the chasm between the vertebrate system and all invertebrate systems. Called “an extraordinary piece of evolutionary exposition,” his theory is based firmly on imaginative speculation about the forces proposed to cause kidney evolution, not the details of how this anatomical evolution could, or did, occur.
Vize adds that knowledge of the kidney has advanced since Smith’s theory was proposed in 1940, “but as a work of integrative logic, and a beautiful piece of writing, it has few rivals” even today. The imaginative speculation of Smith’s theory is illustrated as follows:
This essay begins with the cooling of the earth four billion years ago. The cooling of the crust produced periodic upheavals that had major effects on the earth’s atmospheric conditions, which in turn led to changes in selective pressure. Smith describes how the migration from the oceans to freshwater challenged the physiology of invertebrates and proto-vertebrates and proposes how these pressures were dealt with through the evolution of the kidney. As upheavals drove the early freshwater-inhabiting vertebrates either back to the sea or onto the land, once again physiological barriers had to be overcome, largely by the kidney. (Vize 2004, 344)
How the kidney physically changed and evolved in response to these theoretical climatic upheavals was not explained. Mahasen concluded that
the important factors in the evolution of the basic structure and function of the vertebrate kidney appeared associating with body fluid-regulation, involving the maintenance of a constant water and salt content of the body. . . . the evolution of the vertebrate kidney illustrates how pronephric, mesonephric and metanephric kidneys are represented [by] successful evolutionary responses to the surrounding environmental pressures. (Mahasen 2016, 20)
His review stresses the important factors involved in the evolution of the basic structure and function of the vertebrate kidney were associated with body fluid regulation and the maintenance of constant water and ion content within the body. His version of the evolution of the vertebrate kidney attempts to illustrate the complexity of the system and how pronephric, mesonephric, and metanephric kidneys evolved due to successful evolutionary responses to surrounding environmental pressures. However, even speculation of possible anatomical and physiological steps of kidney evolution are ignored. An extensive literature search did not locate a single theory that proposed details of the evolution of the vertebrate kidney from the contractile vacuoles, or even from the Malpighian tubule system.
Summary
One imaginative scenario proposed to explain that the driving force of kidney evolution was climate change, but the many enormous anatomical and biochemical modifications required as outlined in this paper are ignored. The problem has always been, as is true of all evolutionary proposals, that until all of the parts necessary for a system to function are present and operational, the parts will be selected against. This is especially true of the kidney. This concern applies to all areas of evolution, even basic events, such as the evolution of chordates: “The problem of the origin of the first chordates remains more or less where it was left by the great biologists of the past century—in a sadly unsatisfactory state” (Vize 2004, 344). This problem is especially acute for kidney evolution, an organ critical for life. Life cannot survive without a highly functional kidney and it can function only if all of the necessary parts exist and are integrated with each other as well as the other systems of the body.
Acknowledgements
I acknowledge the help of David Bassett and Kirk Toth in the writing of this paper.
References
Arey, Leslie Brainerd. 1942. Developmental Anatomy: A Textbook and Laboratory Manual of Embryology. Philadelphia, Pennsylvania; and London, England: W. B. Saunders.
Bach, Peter H.; James W. Bridges, and Gilbert H. Mudge. 1985. “Chemically Induced Renal Papillary Necrosis and Upper Urothelial Carcinoma. Part 1.” CRC Critical Reviews in Toxicology 15, no. 3: 217–329.
Beusecum, Justin Van, and Edward W. Inscho. 2015. “Regulation of Renal Function and Blood Pressure Control by P2 Purinoceptors in the Kidney.” Current Opinion in Pharmacology 21 (April): 82–88.
Bradley, T. J. 1985. “The Excretory System: Structure and Physiology.” In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 4. Edited by G. A. Kerkut, and L. I. Gilbert. New York, New York: Pergamum Press.
Brewstera, Ursula C., and Mark A. Perazella. 2004. “The Renin-Angiotensin-Aldosterone System and the Kidney: Effects on Kidney Disease.” The American Journal of Medicine 116, no. 4 (February 15): 263–272.
Buchsbaum, Ralph, et al. 1987. Animals Without Backbones, 3rd ed. Chicago, Illinois: University of Chicago Press.
Clark, Harold W. 1967. Genesis and Science. Nashville, Tennessee: Southern Publishing Association.
Davidheiser, Bolton. 1969. Evolution and Christian Faith. Phillipsburg, New Jersey: The Presbyterian and Reformed Publishing Company.
Davis, Percival, and Dean H. Kenyon. 1989. Of Pandas and People: The Central Question of Biological Origins. Dallas, Texas: Foundation for Thought and Ethics.
Dewar, Douglas. 1957. The Transformist Illusion. Murfreesboro, Tennessee: Dehoff Publications.
Frair, Wayne and Percival Davis. 1983. A Case for Creation, 3rd ed. Chicago, Illinois: Moody Press.
Hickman, Cleveland. P. Jr., Larry S. Roberts, and Allan Larson. 2001. Integrated Principles of Zoology. 11th ed. New York, New York: McGraw-Hill Company.
Jordan, E. L., and P. S. Verma. 2001. Invertebrate Zoology. New Delhi, India: S. Chand and Co.
Kent, George C. 1992 Comparative Anatomy of the Vertebrates. 7th ed. St. Louis, Missouri: C. V. Mosby Company.
Klotz, John W. 1970. Genes, Genesis and Evolution. 2nd ed. St. Louis, Missouri: Concordia Publishing House.
Little, Melissa Helen, and Pamela Kairath. 2017. “Does Renal Repair Recapitulate Kidney Development?” Journal of the American Society of Nephrology 28, no. 1 (January): 34–46.
Mahasen, Laila M. Aboul. 2016. “Evolution of the Kidney.” Anatomy Physiology and Biochemistry International Journal 1, no. 1 (June): 16–21.
Munkácsi, I., and M. Palkovits. 1965. “Volumetric Analysis of Glomerular Size in Kidneys of Mammals Living in Desert, Semidesert or Water-Rich Environment in the Sudan.” Circulation Research 17, no. 4 (1 October): 303–311.
Needham, Joseph. 1930. “The Biochemical Aspect of the Recapitulation Theory.” Biological Reviews 5, no. 2 (April): 142–158.
Ringoir, Severin, Raymond Vanholder, and Shaul G. Massry. 2012. Uremic Toxins. New York, New York: Springer.
Romagnani, Paola. Laura Lasagni, and Guiseppe Remuzzi. 2013. “Renal Progenitors: An Evolutionary Conserved Strategy for Kidney Regeneration.” Nature Reviews Nephrology 9, no. 3 (March): 137–146.
Romer, Alfred Sherwood. and Thomas S. Parsons. 1986. The Vertebrate Body. 6th ed. Philadelphia, Pennsylvania: Sanders College Publishing.
Shute, Evan. 1971. Flaws in the Theory of Evolution. Nutley, New Jersey: Craig Press.
Smith, Homer W. 1953. From Fish to Philosopher. Boston, Massachusetts: Little, Brown and Company.
Speck, Patricia L. 1994. “The Kidney: A Designed System for Plasma Homeostasis.” In Proceedings of the Third International Conference on Creation. Edited by Robert E. Walsh, 505–511. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Valverde-Islas, Laura E., Esteban Arrangoiz, Elio Vega, Lilia Robert, Rafael Villanueva, Olivia Reynoso-Ducoing, Kaethe Willms, Armando Zepeda-Rodriguez, Teresa I. Fortoul, and Javier R. Ambrosio. 2011. “Visualization and 3D Reconstruction of Flame Cells of Taenia solium (Cestoda).” PLoS One 6. no. 3 (March 11): e14754.
Vize, Peter D. 2004. “A Homeric View of Kidney Evolution: A Reprint of H. W. Smith’s Classic Essay With a New Introduction.” The Anatomical Record, 277A, no. 2 (April): 344–354.