The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
The evolutionary claim is that the one-chambered heart pump evolved first, and then it evolved into the two-chambered heart, then the three-chambered heart, and finally, the four-chambered heart, which is used in all mammals. In short, evolution postulates that the circulatory system evolved from a simple system in bacteria to the complex system in humans by the natural selection of billions of mutations (Bishopric 2005).
However, the major insurmountable problem is that each type of heart is an integral part of a specifically designed complex circulatory system. To function properly, each type of heart and circulatory system works together and very differently from all the others. As a result, how one type of system evolved into the next type has baffled Darwinists. Not even plausible “just-so” stories have been proposed to explain how one heart system evolved into a very different system. Evolution is unable to explain their origin.
Keywords: Heart, heart evolution, heart design, human evolution, irreducible complexity, intelligent design
Introduction
Two basic heart designs exist, an open system and a closed system which uses the chambered heart design. Evolutionists postulate that the chambered heart evolved from the open system and that each chambered heart evolved to add one more chamber until the four-chambered heart was achieved.
The Open System
The simplest circulatory system is called an “open system,” in contrast to a “closed system.” In an open system, such as that used in insects, cells and fluids freely circulate in some liquid medium most everywhere in the animal body (Wilder 1909, 317). Instead of blood, the extant open-circulatory systems contain hemolymph, that is, respiratory proteins similar to hemoglobin. Instead of the complex system of veins and arteries used in the closed system, organisms with open circulatory systems have a central body cavity called a hemocoel. This cavity exists inside most invertebrates in a location where both digestive and circulatory functions are performed.
This hemocoel may have “artery”-like structures to help guide the blood hemolymph around the body tissues—but these systems are open and cannot circulate blood as rapidly as does a closed, muscle-assisted arterial system.
Spiders, like most arthropods, have an open circulatory system. Their bodies are filled with hemolymph, which is pumped by a one-chambered “heart” into spaces called sinuses that surround their internal organs. Called a dorsal vessel, this “heart,” is located in the abdomen and is not divided into chambers, but rather consists of a simple tube that contracts.
Most mollusks (including snails, clams, mussels, squid, and octopods), have an open circulatory system, yet employ a very different heart design than insects. One example of how different the mollusk heart is from the closed-chambered heart is that their heart atria also function as part of their excretory system. After filtering waste products out of the blood, the heart atria dumps the waste into the coelom as urine.
The Chambered Heart
The “chambered heart is an amazing oddity” used in only two groups of animals: vertebrates and mollusks (Xavier-Neto and Carvalho 2021, 39). Four types exist: one-, two-, three-, and four-chambered hearts (fig. 1). Actually, each type of heart is specially designed to function with only one of the four types of circulatory systems. Thus, it is more accurate to speak of four circulatory-system designs instead of four-chamber heart designs. Furthermore, each type was designed to function in a specific kind of animal. The one-chambered design is used in insects, the two-chambered in fish, the three-chambered in most reptiles, and the four-chambered in most mammals and birds.
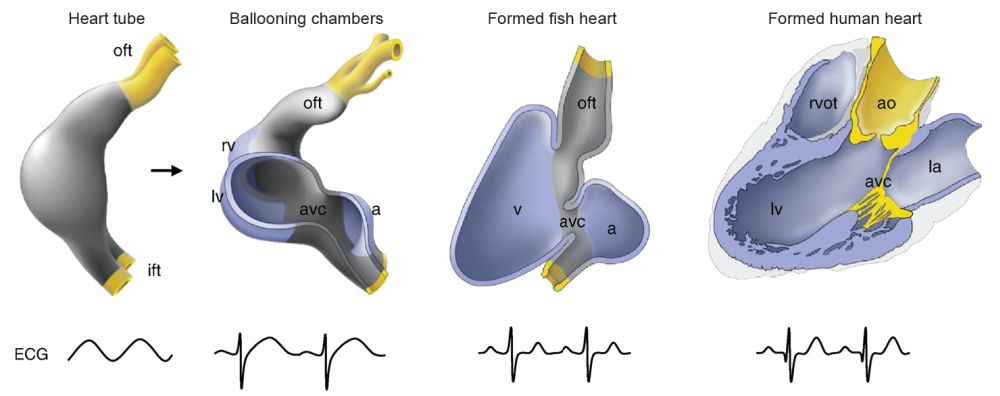
Fig. 1. Heart anatomy comparison between fish, amphibian, reptile, bird, and mammal. From Wikimedia Commons. https://ars.els-cdn.com/content/image/1-s2.0-S0167488912002868-gr2_lrg.jpg. Key: oft = outflow ; ift = inflow; rv = right ventricle; lv = left ventricle; avc = atrioventricular canal; a = atrium; v = ventricle; rvot = right ventricular outflow; ao = aorta; la = left aorta.
To evolve a one-chambered-heart type into another requires major changes in everything from the veins and arteries surrounding the heart to the interior heart design. It also requires a major redesign of the artery and venous circuit that interacts with the lungs, as well as other structural changes in the heart walls and valves. Furthermore, “chambered hearts in vertebrates and mollusks cannot be traced back to a common origin in a more primitive clade of animals (i.e., a homology due to the presence of a chambered heart in a common ancestor [is lacking]). Thus, there are arguments in favor of a completely independent evolution for vertebrate and mollusk hearts (evolutionary convergence), or in support of a parallel origin in a distant ancestor” (Xavier-Neto and Carvalho 2021, 39). Another study using comparisons of hearts used in aquatic and terrestrial life-styles determined the changes required, but admitted “little is known about the exact shifts linked with this rearrangement” (Olejnickova, et al. 2021).
Changes required to evolve the four-chambered heart from the three-chambered design include the development of a forked abdominal aorta into a single aorta and a major alteration in the heart septum in order to create a separate ventricle chamber. For an animal to survive, these changes must be made in such a way that the heart can effectively function while the heart is evolving until the many major alterations are completed.
A literature search of published articles in peer-reviewed journals on the evolution of chambered hearts reveals that no one has even attempted to outline how the many major changes could occur while allowing the animal to survive during the transition from one heart design into another heart type.
One study admitted that while “currently there are no reasons to doubt that chambered hearts originated from peristaltic pumps, the actual sequence of events is not clear” (Xavier-Neto and Carvalho 2012, 39). They then outlined three very different possibilities, namely the sequential hypothesis, the recruitment hypothesis, and the patterning hypothesis, all of which are very hypothetical and contradictory. Another heart study, this one of the zebra fish heart development, noted its development will be explained by some “yet-to-be revealed mechanisms and evolutionary origins” (Kemmler et al. 2021, 8). Many other similar studies exist involving the heart, all of which were consulted and revealed a similar lack of viable theories on heart origins and evolution (Salazar-Ciudad 2006).
Chambered Heart Details
The two-chambered fish heart draws in deoxygenated blood from the body into a single atrium. This blood is then forced into the much larger ventricle that pumps it out to the body cavity. This system is termed a “single circulation” design because blood first enters the heart, is then pumped through the gills to be oxygenated, and, lastly, is forced out into the body in one circulation cycle.
Experts can only guess when, how, or in what lineage the alleged transition from the two-chambered fish heart to the three-chambered amphibian heart occurred. This is a very difficult transition to even create “just-so” stories to explain, as is commonly done by evolutionists. A three-chambered heart has “double circulation” and is irreducibly complex with respect to the “double circulation” design. The two-chambered hearts of fishes have “single circulation” and the basic design is very different from a three-chambered heart.
The four-chambered heart system uses a dual circulatory system, namely the pulmonary and systemic circulatory systems. This double circulatory system uses two separate circuits requiring the blood to pass through the heart twice. It travels once through the pulmonary circuit, which is located between the heart and lungs, and the second time through the systemic circuit, located between the heart and all other body organs. In the single circulation system used in fishes, blood passes only once through the heart for each complete circuit in the body.
Although most reptiles have a three-chambered heart, two atria, and a semi-separate ventricle (for example, snakes, turtles, lizards) some reptiles, (the crocodilians which include crocodiles, alligators, caimans and gavials), as well as birds, have a four-chambered heart (two atria and two ventricles), as do mammals (Meyer et al. 2007, 132) (see Appendix, fig. 2).
The crocodilians case does not solve the problem of how the four-chambered heart first evolved in mammals as some speculate. The reasons including how the three-chambered heart evolved into the four-chambered heart still must be answered (Meyer et al. 2007, 132). Secondly, the crocodilians’ four-chambered heart employs a unique design which makes it as different from the mammal’s four-chambered heart as it is from the reptiles three-chambered heart (Franklin and Axelsson 1994; Franklin et al. 2000). This design included a shunt that allows it to redirect blood back though its tissues instead of to the lungs into the body when it is in no danger (Cook et al. 2017). Another problem is the crocodilians are not part of the mammal-like reptiles thought to be ancestral to the first mammals.
In summary, three- and four-chambered hearts use a pulmonary circuit to oxygenate blood, that is, the heart forces blood from the heart to the lungs and back to the heart (fig. 1). When oxygenated, the blood is then pumped out of the heart and into the body (the systemic circuit). The tubes used in a closed system must be able to carry oxygenated blood to the entire body. This requires a feedback system to cause vasculogenesis, that is, the differentiation of precursor angioblast cells into endothelial cells, and the de novo formation of the primitive vascular network. Angiogenesis, the growth of new capillaries from pre-existing blood vessels, is also necessary, beginning in the embryo and continuing as an adult to maintain and repair the system.
This process is triggered by cells not sufficiently supplied with nutrients, especially glucose, and which produce (or cause nearby cells to produce) the growth factors that stimulate the formation of new blood vessels (Maragoudakis, Gullino, and Lelkes 2012). This process is used in all organisms that have multiple heart chambers. One of the most well-studied angiogenesis factors is vascular endothelial-derived growth factor (VEGF). VEGF and other angiogenesis factors produced by cells cause the “evolution”, (meaning development within the organism), of blood vessels that feed the cells (Muñoz-Chápuli 2011). The VAGF system is yet another contrast between invertebrates and vertebrates, the latter being much more complex and more tightly regulated (Kipryushina, Yakovlev, and Odintsova 2015).
A repair system is also required when the blood vessels are ruptured or injured, which is inevitable. Lacking this system, bleeding out is a major concern. When a blood vessel is injured or ruptured, thromboxane A2 and other hormones, along with local nervous reflexes, cause the reconstruction of the ruptured vessel, very rapidly reducing blood leakage (Joshi, Nandedkar, and Mendhurwar 2017, 184). Next, platelets, which are normally repelled by the intact blood vessel wall, become sticky and adhere to the collagen fibers of the vascular surface, plugging the vascular opening. Soon the clot begins to contract, causing a reduction in leaking plasma and repair to begin. This process is not required in an open circulatory system, but must exist in all organisms that have a multi-chambered heart.
To help explain the enormous changes required for heart/circulation evolution, evolutionists postulate long ages, (such as the four-chambered heart first evolved 0.5 billion years ago), to blunt the enormity of changes required to convert a two-chambered heart to a three-chambered heart and eventually into a four-chambered heart.
The Heart is Part of an Irreducibly Complex System
A major problem with all heart evolution claims is the fact that the entire circulatory system is irreducibly complex (see Appendix, fig. 3). At least three subsystems are all required:
- an organ (lungs/gills) for enriching the hemophlegm (blood) with oxygen,
- a complex network of closed tubes to carry the energy-rich blood to the body (veins and arteries), and
- a pumping mechanism (heart) to transport energy-rich fluid throughout the body.
All of the systems described above must simultaneously exist for a chambered heart to function. Evolutionists attempt to get around this fact by imagining scenarios where some, or all, of the subsystems could somehow originate and function as freestanding structures functioning independently. Then, after millions of years, they began to work together forming the complex system existing today.
However, a closed-tube network lacking a pumping mechanism to transport fluid is useless, as is a pump without fluid or tubing. Oxygen exchange occurs in many organisms through the skin without a chordate-like circulatory system, but no advantage exists for such an organism to randomly mutate until a single oxygen exchange organ (the lungs/gills) exists. Once a single oxygen-exchange organ existed, to then function required the transport network provided by the heart, veins, and arteries. Scenarios attempting to explain the evolution of the circulatory system step-by-step consistently fail—which is why they are not found in the literature.
The overall complexity of the system is illustrated by the fact that the 11-ounce human heart pumping machine, the size of a human fist, pumps 2,000 gallons of blood a day through 60,000 miles of blood vessels. The total length of the arteries, veins, and capillaries is 62,000 miles (100,000 km), or nearly enough to go 2.5 times around the earth. Over the course of the average human lifetime, the heart beats over 2 billion times and pumps over 100 million gallons of blood (Dev 2016). It requires a carefully balanced interior network of tubes, holes, and valves which keep fluid constantly flowing in the right direction both in and out of the respiratory system. Miles of fluid-directing parts and a strong and properly shaped pumping muscle, the hardest working muscle in the body, are required for it to function (Dev 2016, 40). The existing positioning of the parts, as in any intelligent design, is a good example of specified complexity. Also, both circuits, the systemic and pulmonary, must be perfectly balanced in pressure dynamics or a mismatch and failure will result.
Evolutionists Attempt to Explain Heart Evolution
Evolutionists usually address the problem of heart evolution by first outlining the working of the four types of hearts, as shown in fig. 1. Then, typically, they explain that one system evolved into another system without attempting to explain how this occurred or if this is even possible. For example, it is simply stated as fact that a three-chambered heart evolved into a four-chambered heart, then a Darwinian date is given when this event was supposed to have occurred. This approach was used by Bishopric (2005), Simões-Costa et al. (2005), and Stephenson, Adams, and Vaccarezza (2017). Of all the accounts of heart evolution I reviewed, not even “just-so” stories were attempted to explain how the hearts evolved.
For example, Professor Bishopric noted that a “critical step in evolution was of a single or paired primordium . . . most likely a tubular, pulsatile structure that lacked an enclosed vascular system . . . lacking chambers, septa, and valves” (Bishopric 2005, 19). From there she speculated that gradualism slowly, over many millions of years, evolved more advanced hearts until the apex design existing today was reached, namely the four-chambered human heart (Bishopric 2005, 24–25).
She does acknowledge the many problems with heart evolution scenarios, even about basic issues, noting that, for example, “Whether the insect and vertebrate hearts evolved independently has been argued for nearly 200 years” (Bishopric 2005, 19). Nonetheless, no hard evidence was presented to support heart evolution. Her review was largely speculation that ignored all of the tough problems in this field that were discussed above.
Another attempt to explain heart evolution was by Simões-Costa et al., who, instead of citing empirical evidence, “suggested” possible scenarios, such as that the heart
chambers arose in evolution through patterning of cardiac precursors by signaling events . . . . Difficulties in ascribing chamber identity to cardiac compartments arise because during vertebrate evolution, cardiac segments may have regressed, merged into others, or divided into left, right or more compartments. Besides, chambers can be defined on either morphological . . . or functional grounds. At some point in evolution the precursors of these cells were patterned in the AP axis, generating all or a subset of chamber-specific myocytes. We suggest that this event made possible a later morphogenetic reorganization that created at once, two, perhaps more, cardiac chambers. (Simões-Costa et al. 2005, 2, 10, 13; emphasis added)
This major anatomical alteration which is required to evolve the four heart types was explained as follows: “evolutionary gene mutations ensured that the anatomy of the vertebrate heart became more complex” (Stephenson, Adams, and Vaccarezza 2017, 788). The problem is that it
is very difficult to conceive mutations occurring early in the development of a creature [that] could produce beneficial large-scale change. Even though some mutations do occur early in the development of some organisms, these mutations inevitably disrupt the orderly process of organ system construction. Evolutionists believe that fish evolved into amphibians that evolved into reptiles that evolved into mammals. The problem is that fish have a two-chambered heart; reptiles have a three-chambered heart; and mammals have a four-chambered heart. Since Darwinian evolution requires multiple, small changes that eventually result in large-scale improvements, any offspring with even minor changes to their heart would most likely not function as a fully developed heart. Any offspring with any significant change to their heart would have a weaker and less efficient heart and therefore would have a more difficult time of survival. (DeBenedictis 2014, 251)
For these and other reasons, the heart and the entire circulatory system are not gradually evolvable in a step-by-step Darwinian manner.
Conclusions
Although it is widely believed that the four-chambered heart-circulation system evolved from a three-chambered heart by mutations, the literature is not only void of plausible evidence, but I was unable to locate even viable attempts at “just-so” stories. The theories that were discussed focused on a review of the common scenario of the first life-form evolving to humans, assuming it to be true, then proclaiming therefore that heart evolution by gradual steps from a pulsating tube to a four-chambered heart is also true. It is then rationalized that this evolution cannot be independently documented because it occurred many billions of years ago.
Or, as explained by Schmidt-Rhaesa, as an animal evolves, the diffusion process “becomes a problem with growing size and complexity. Therefore, many animals have invented some kind of circulatory system” (Schmidt-Rhaesa 2007, 191; emphasis added). Since evolution is true, the reasoning goes, the gradual development of the heart-driven circulatory system must also be true (Schmidt-Rhaesa 2007, 1). Notably, this reference mentions problems with the evolution of organ systems some 57 times.
The fact is that even the evolution “from a 2 to a 3 chambered heart requires a lot more than the mere duplication of a chamber, but a complete reworking of the veins and arteries surrounding the heart, interior valves of the heart, and the creation of this interior circuit which can properly interact with the lungs” (IDEA Center 2004). It is more credible, as taught in Genesis, that a supernatural God spoke into existence life, and all that is required for life to live, rather than that it evolved out of nothing, as evolution teaches.
References
Bishopric, Nanette H. 2005. “Evolution of the Heart From Bacteria to Man.” Annals of the New York Academy of Sciences 1047, no. 1 (June): 13–29.
Cook, Andrew C., Vi-Hue Tran, Diane E. Spicer, Jafrin M. H. Rob, Shankar Sridharan, Andrew Taylor, Robert H. Anderson, and Bjarke Jensen. 2017. “Sequential Segmental Analysis of the Crocodilian Heart.” Journal of Anatomy 231, no. 4 (August 1): 484–499.
DeBenedictis, Albert. 2014. Evolution or Creation? A Comparison of the Arguments. 3rd ed. Bloomington, Indiana: Xlibris.
Dev, N. Prabhu. 2016. Human in Person: One Life to Live. India: Partridge Publishing.
Franklin, Craig E., and Michael Axelsson. 1994. “The Intrinsic Properties of an in situ Perfused Crocodile Heart.” Journal of Experimental Biology 186, no. 1 (January): 269–288.
Franklin, Craig, Frank Seebacher, Gordon C. Grigg, and Michael Axelsson, 2000. “At the Crocodilian Heart of the Matter.” Science 289, no. 5485 (8 September): 1687–1688.
IDEA Center. 2004. “The Vertebrate Animal Heart: Unevolvable, Whether Primitive or Complex.” Intelligent Design and Evolution Awareness Center. http://www.ideacenter.org/contentmgr/showdetails.php/id/1113.
Joshi, Vijaya D., Ashalata N. Nandedkar, and Sadhana S. Mendhurwar. 2017. 3rd ed. Anatomy and Physiology for Nursing and Healthcare. New Delhi, India: BI Publications.
Kemmler, Cassie L., Fréderike W. Riemslagh, Hannah R, Moran, and Christian Mosimann. 2021. “From Stripes to a Beating Heart: Early Cardiac Development in Zebrafish.” Journal of Cardiovascular Development and Disease 8, no. 2 (February 10): 17.
Kipryushina, Yulia O., Konstanti V. Yakovlev, and Nelly A. Odintsova. 2015. “Vascular Endothelial Growth Factors: A Comparison Between Invertebrates and Vertebrates.” Cytokine and Growth Factor Reviews 26, no. 6 (December): 687–695.
Maragoudakis, Michael E., Pietro Gullino, and Peter I. Lelkes. 2012. Angiogenesis in Health and Disease. New York, New York: Springer.
Meyer, Stephen C., Scott Minnich, Jonathan Moneymaker, Paul A. Nelson, and Ralph Seelke. 2007. Explore Evolution: The Arguments for and Against Neo-Darwinism. London, United Kingdom: Hill House Publisher.
Muñoz-Chápuli, Ramón. 2011. “Evolution of Angiogenesis.” International Journal of Developmental Biology 55, nos. 4–5: 345–351.
Olejničkova, Veroniká, Hana Kolesova, Martin Bartos, David Sedmera, and Martina Gregorovicova. 2021. “The Tale-Tell Heart: Evolutionary Tetrapod Shift From Aquatic to Terrestrial Life-Style Reflected in Heart Changes in Axolotl (Ambystoma mexicanum).” Developmental Dynamics (23 August): 1–11. https://anatomypubs.onlinelibrary.wiley.com/doi/abs/10.1002/dvdy.413.
Salazar-Ciudad, I. 2006. “On the Origins of Morphological Disparity and Its Diverse Developmental Bases.” BioEssays 28, no. 11 (November): 1112–1122.
Schmidt-Rhaesa, Andreas. 2007. The Evolution of Organic Systems. New York, New York: Oxford University Press.
Simões-Costa, Marcos S., Michelle Vasconcelos, Allysson C. Sampaio, Roberta M. Cravo, Vania L. Linhares, Tatiana Hochgreb, Chao Y. I. Yan, Brad Davidson, and José Xavier-Neto. 2005. “The Evolutionary Origin of Cardiac Chambers.” Developmental Biology 277, no. 1 (January 1): 1–15.
Stephenson, Andrea, Justin W. Adams, and Mauro Vaccarezza. 2017. “The Vertebrate Heart: An Evolutionary Perspective.” Journal of Anatomy 231, no. 6 (December): 787–797.
Wilder, Harris Hawthorne. 1909. History of the Human Body. New York, New York: Henry Holt.
Xavier-Neto, Jose, and Ismar de Souza Carvalho. 2021. “Paleontological Treasures Among Commonplace Fossils: A Paradigm to Study Evolutionary Innovation.” In Ancient Fishes and their Living Relatives: A Tribute to John G. Maisey, edited by Alan Pradel, John S. S. Denton, and Philippe Janvier, 37–47.
Appendix: The Contrast Between the Different Heart Designs: Explanation of Fig. 3
The heart’s main function is to move blood throughout the body. To achieve this purpose it regulates the body’s blood pressure and heart rate. The nervous system sends signals to speed up or slow down the heart rate. The endocrine system releases hormones to constrict or relax blood vessels to also regulate blood pressure. Thyroid hormones can also regulate heart rate.
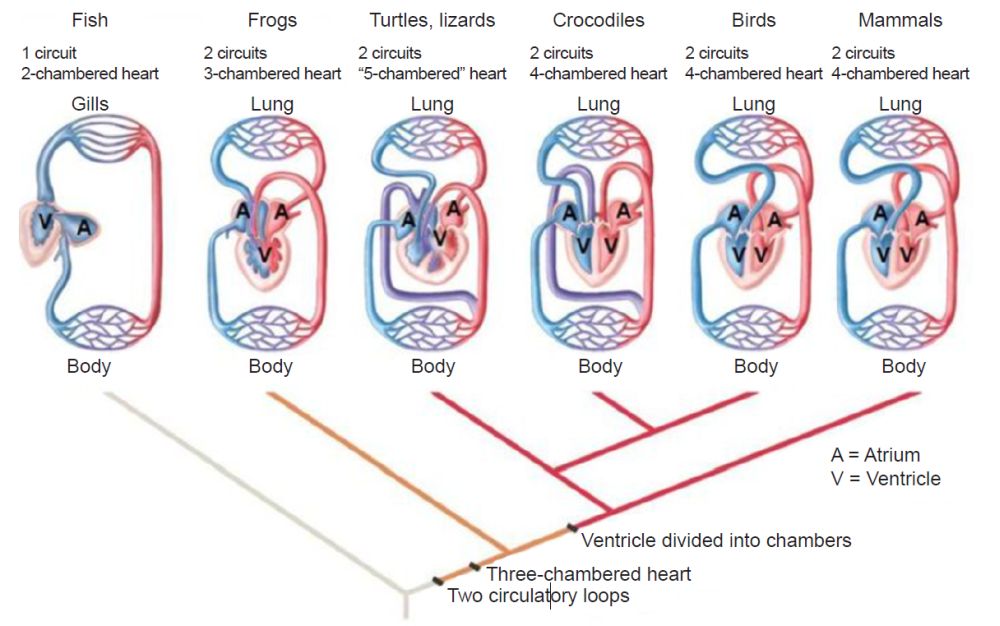
Fig. 2. A comparison of the basic heart designs from a two- to a four-chambered design. From Wikimedia Commons. https://www2.nau.edu/lrm22/lessons/heart_evolution/heart_evolution.html.
The muscular atrioventricular septum divides the heart walls into the left and right chambers. The heart walls consist of the inner layer (endocardium), the muscular middle layer (myocardium), and the protective outer layer (epicardium). The protective sac that covers the entire heart, the pericardium, produces fluids to lubricate the heart and prevent it from rubbing against other organs. The heart is divided into four chambers, two on the top (atrium, plural atria) and two on the bottom (ventricles), one on each side of the heart.
Two large veins deliver oxygen-poor blood to the right atrium. The superior vena cava carries blood from the upper body, and the inferior vena cava from the lower body. Then the right atrium pumps blood to the right ventricle, which pumps oxygen-poor blood to the lungs through the pulmonary artery. After the lungs oxygenate the blood, the pulmonary veins carry it to the left atrium which pumps it to the left ventricle, which then sends oxygen-rich blood to the rest of the body.
The heart valves allow blood to flow to the next location, and prevent it from flowing backward. The atrioventricular (AV) valves are between the upper and lower heart chambers. The tricuspid valve is between the right atrium and right ventricle, and the mitral valve is between the left atrium and left ventricle. The semilunar (SL) valves open when blood flows out of your ventricles. The aortic valve opens when blood flows out of the left ventricle to your aorta which carries oxygen-rich blood to the body. The pulmonary valve opens when blood flows from the right ventricle to the pulmonary arteries, the arteries that carry oxygen-poor blood to the lungs.
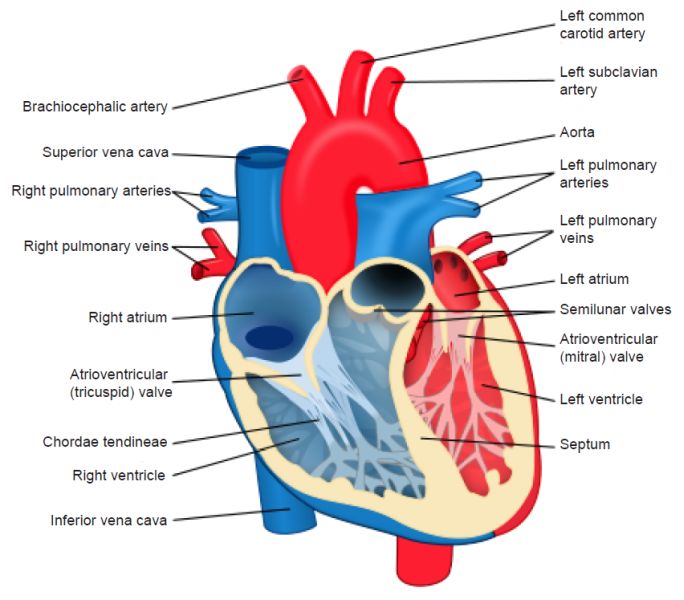
Fig. 3. The four-chambered heart. Notice the four chambers, the valves, and the blood vessels allowing it to function. From Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/thumb/e/e0/Heart_diagram-en. svg/512px-Heart_diagram-en.svg.png.
The heart pumps blood through three blood vessel types. Arteries carry oxygen-rich blood from the heart to the body’s tissues, (except the pulmonary arteries, which move blood to the lungs). Veins carry oxygen-poor blood back to the heart, and capillaries are small blood vessels that allow movement of oxygen-rich blood into the cells.
The heart receives nutrients through a network of coronary arteries that transverse along the heart’s outer surface. The electrical conduction system that controls the rhythm and pace of the heartbeat includes the sinoatrial (SA) node which sends the signals that cause the heart to beat. The atrioventricular (AV) node carries electrical signals from the heart’s upper chambers to the ventricles below to coordinate the heartbeat.
The network of electrical bundles and fibers includes: the left bundle branch block which sends electric impulses to the left ventricle. The right bundle branch block which sends electric impulses to the right ventricle. The bundle of His sends impulses from the AV node to the Purkinje fibers which cause the heart ventricles to contract to pump out blood.