Research conducted by Answers in Genesis staff scientists or sponsored by Answers in Genesis is funded solely by supporters’ donations.
Abstract
Biosystematics is in great flux today because of the plethora of genetic research which continually redefines how we perceive organism relationships. Despite the large amount of data being published, the challenge is having enough knowledge about genetics to draw conclusions regarding the biological history of organisms and their taxonomy. Based on the analyses of extant organism molecular data, taxonomy, and hybridization capability a tentative estimate of one tuatara, two amphisbaena, and 41 snake kinds is proposed as being on the Ark.
Keywords: Ark Encounter, biosystematics, taxonomy, amniotes, reptiles, snake, amphisbaena, tuatara, lepidosaurs, squamata, serpentes, kind, baraminology
Introduction
Creation research is guided by God’s Word, which is foundational to the scientific models that are built. The Ark Encounter Project has tasked creation researchers to investigate several questions surrounding what the kind is, how to recognize it, and the mechanisms involved with organism diversification (Brophy and Kramer 2007; Lightner et al. 2011; Sanders and Wise 2003; Turner 2009; Williams 1997; Wise 1992; Wood et al. 2003; Wood 2003; Woodmorappe 1996).
In previous papers the numbers of amphibian, crocodile, and turtle kinds were estimated (Hennigan 2013a, 2013b; Hennigan 2014). The purpose of this paper is to use all available information in order to make an initial estimate of the identification and numbers of extant Lepidosaur kinds, except for the “lizards.” This includes tuataras, amphisbaenas, and snakes. Lizards will be the subject of a future paper.
The State of Biosystematics and Taxonomy Today
Biosystematics is the science of discovering, classifying, and organizing biological diversity. The science of identifying taxa and naming organisms is taxonomy. There is no universally accepted procedure for organism classification and currently these disciplines are in great flux as researchers are placing more importance on the accumulating new genetic and molecular data for phylogeny development; much is being changed accordingly. Therefore, how organisms are named and organized today may change tomorrow, depending on the data and assumptions about that data. For example, naturalists assume randomness and universal common descent. In keeping with these assumptions, they are gradually moving away from Linnaean hierarchies and toward the PhyloCode system (Vitt and Caldwell 2009, pp. 20–25). In contrast, creation biologists recognize the God of Scripture as the Creator of all “kinds” and assume “forest”, rather than “tree” thinking, when interpreting biological diversity and disparity. Instead of the tree (or trees) of life that represent evolutionary random processes and universal common descent, the creationist may visualize individual trees in a forest as the originally created kinds. The separation of each tree represents the discontinuity between kinds and the degree of branching represents limited descent with modification within the kind over time. Specifically, creationists are interested in how creatures have diversified from the originally created baramins and the archetypes that left the Ark. While genetic and molecular data will be incorporated in this taxonomic analysis, there is still not enough knowledge about biochemistry to draw conclusions regarding the biological history and taxonomy of organisms. Therefore, other variables will also be incorporated such as hybridization data (Genesis 1; Genesis 7; Lightner et al. 2011; Sanders and Wise 2003; Wood 2006a, 2006b).
It is important to consider the following precautions and perspectives. Baraminologists tend to equate kinds with the family and for many cases with good reason (Wood 2006a). However, we should carefully analyze the structures, behaviors, and physiologies of members of a putative kind and look at the genetic reasons why a certain member of a kind doesn’t have characters that the other members possess (Wilson 2010). This research is meant to be a foundation upon which further research and understanding of God’s diverse organisms can be built. Within his Trinitarian character God is diverse and we would expect that his creation would reflect that diversity in his creatures in aspects such as genetics, species, populations, communities, and ecosystems. When we better understand what mechanisms are involved in the production of differences, we should be better able to infer whether they are traits produced by direct creation, post-Flood diversification through unknown designed genetic mechanisms, and/or random mutations.
The Non-Avian Reptiles
Extant reptiles consist of the following taxa: birds, turtles, tuataras, snakes, amphisbaenas, lizards, and crocodiles (Pough et al. 2004 p. 8; Vitt and Caldwell 2009, p. 24). Currently 9904 species of non-avian reptiles have been identified, with 104 new species described this year alone (Uetz 2013). Birds are the subject of another paper (Lightner 2013). Reptiles, along with mammals, are amniotes and have an amniotic membrane that encloses the embryo in a fluid-filled sac. Based on current taxonomy, all extant reptiles, with the possible exception of the turtles, are classified in Diapsida. All extant diapsids are classified in the Sauria taxon. Sauria is further subdivided into the Archosauromorpha (crocodiles, dinosaurs, birds, and, depending on the researcher, turtles) and Lepidosauromorpha (tuataras, lizards, amphisbaenas, and snakes [Vitt and Caldwell 2009, p. 19]). The rest of this paper will focus on extant Superorder Lepidosauria, excluding lizards.
Superorder Lepidosauria
Lepidosaurids, based on Linnaean taxonomy, are currently classified accordingly: Class Reptilia; Subclass Diapsida; Superorder Lepidosauria; and two Orders: Rhyncocephalia (Suborder Sphenodontida—tuataras) and Squamata (“lizards” that also include snakes and amphisbaenas). Assuming the evolutionary naturalist paradigm, snakes (Clade Ophidia—Suborder Serpentes) and amphisbaenas (Suborder Amphisbaenia) are considered monophyletic groups of lizards and are nested within the lizard taxon (Suborder Lacertilia [Uetz 2013]). Squamates have the greatest reptile diversity of all reptile taxa and representatives can be found on all continents except Antarctica (Uetz 2013; Vitt and Caldwell 2009, pp. 4, 514–515).
All lepidosaurs share many characters including: a transverse cloacal opening, distally notched tongue used for catching prey, full body ecdysis (skin shedding), imperforate stapes, teeth attached superficially to the jawbone (acrodont teeth on the surface of the jaw or pleurodont teeth on the inner side of the jaw), and fused pelvic bones on adults (Vitt and Caldwell 2009, p. 513). What follows are brief descriptions of each group, the estimated kinds, and average snout to vent length (SVL) or average total length (TL) ascertained from Uetz (2013) and Vitt and Caldwell (2009).
Tuatara: Order Rhyncocephalia
Suborder Sphenodontida
1. Tuatara kind—
Family Sphenodontidae
Subfamily Sphenodontinae
1 genus (Sphenodon)—formerly two species S. punctatus and S. guntheri, but is now considered monotypic—SVL = 23 cm (9 in)

Source: http://en.wikipedia.org/wiki/Tuatara.
Fig. 1. Sphenodon punctatus.
The word tuatara comes from a Maori word that means “spiny back” (Helder 1991; Kiwi Conservation Club 2013). They are known for their well-developed parietal eye, also referred to as a third eye or pineal eye (also found in other squamates) which is photoreceptive and associated with biological cycles and thermoregulation (Vitt and Caldwell 2009, p. 79). Found only on small islets off the coast of New Zealand, the one species of tuatara is all that is left of at least 16 genera that are now extinct (Uetz 2013). Hypotheses vary as to why they have minimal diversity and so few have survived, but suggestions include: lizards outcompeting them, rat predators eating both young and eggs, and/or an inability to meet changing environmental demands after the Flood. Although they look like squamates, they have several unique characters not shared with them: a narrow quadrate bone (back of lower skull) with greatly reduced or lateral concha (shell-shaped bone structures of the skull); enclosed or partially enclosed temporal fenestra (hole in the skull behind the eye); jugal bone of skull touching the squamosal bone posteriorly; coronoid process is prominent on the mandible; several enlarged anterior palatine, mandibular, and dentary teeth; and premaxillary teeth are replaced with chisel-like extensions where the upper beak overhangs the lower jaw (the reason for their local name, the “half beaks”—Vitt and Caldwell 2009, p. 513). Unique traits include the ability to delay hatching for of up to 16 months after the eggs are laid and having the lowest known optimal incubation temperatures (18–22°C) of extant reptiles (Vitt and Caldwell 2009, pp. 513–514).
Until recently, two species were recognized, however Hay et al. (2010) analyzed microsatellite DNA, mtDNA, and allozyme data and concluded that genus Sphenodon should be considered a single species (Uetz 2013). Because of their differences with Squamata, I include the tuatara as a separate kind.
Amphisbaena: Order Squamata
Suborder Amphisbaenia
Worm lizards and two-legged worm lizards, Suborder Amphisbaenia, consist of six families and 184 species (Uetz 2013). Characters shared include round body in cross section (Trogonophiidae is an exception having a body with a triangular cross section), reduced right lung (in snakes it is often the left lung that is reduced or absent), annulated bodies made of rectangular shaped scales that are juxtaposed to each other throughout the length of the organism, limbless in most except for Bipedidae, occasional pelvic “vestiges” in most, autotomous tail that does not regenerate if broken (in monotypic Rhineuridae the tail is short and lacks autotomy), filamentous papillae on tongue that is covered by lingual scales in diagonal rows, outer ears absent, eyes covered with skin and scales, movement is accordion-like, ability to move backwards and forwards, carnivorous, and either oviparous or ovoviviparous (Vitt and Caldwell 2009, pp. 528–531).
They can be found from the southeastern USA and Central/South America to Africa and Eurasia. The two-legged worm lizards (Bipedidae—Bipes sp.) from coastal southwestern Mexico and Baja California are unusual because they have mole-like forelimbs. All amphisbaenas are fossorial (Vitt and Caldwell 2009, p. 530). The Florida worm snake, (Rhineuridae—Rhineura floridana) is the only member of the family and it inhabits mostly Florida, though there have been two reports of sightings in Georgia (Jensen et al. 2008). No hybridization data was located for this suborder. Overall the variability is well within that which is found intraspecifically, including squamate oviparity and ovoviviparity (Handwerk 2010; Lightner 2008). Though the suborder is probably a holobaramin, Bipedidae, because of their two legs and mole-like forelimbs, will be identified as a separate kind so numbers are not underestimated.
2. Worm Lizard kind
5 Families:
Amphisbaenidae (11 genera, 167 species),
Blanidae (1 genus, 5 species),
Cadeidae (1 genus, 2 species),
Rhineuridae (monotypic), and
Trogonophiidae (4 genera, 6 species)
SVL = 21 cm (8 in)

Source: http://www.wildflorida.com. Photograph: Fiona Sunquist.
Fig. 2. Rhineura floridana.

Source: http://en.wikipedia.org/wiki.
Fig. 3. Blanus cinereus.
3. Two-Legged Worm Lizard Kind
Family Bipedidae
1 genus, 3 species
SVL = 18 cm (7 in)

Source: http://en.wikipedia.org/wiki.
Fig. 4. Bipes biporus.
Snakes: Order Squamata
Suborder Serpentes (Clade Ophidia)
Presently there are 3451 species of snakes recognized in 24 families and they are the second most speciose group of reptiles (Uetz 2013). At least 19 new species were described this year (Uetz 2013). They are considered a monophyletic group of lizards and are quite diverse in their morphology, behavior, and habitat preference. Some species are fully aquatic while others are fully subterranean. Some can glide from tree to tree while others contain some of the most powerful venoms found in creation. A couple of species such as the Eastern hognose snake (Heterodon platirhinos) will shuffle through several bizarre behaviors when disturbed, from spreading its head like a cobra and rattling its tail in leaves, to “bleeding” from the mouth and feigning death (Hennigan 1998).
The Problem of Biological Evil
Within Christian theism, if all things were created very good, the issue of disease, death, and suffering (e.g. biological evil) can be used as an objection to the acceptance of a biblical worldview or lead to wrong conclusions about the goodness of God. This theological issue is in the realm of theodicy and deals with the question of why a loving God would design organisms, such as venomous snakes, with superb toxic chemicals and killing apparati. Man’s disobedience and God’s subsequent curse on this planet is certainly the starting point of such a discussion. Though space does not allow for an in-depth coverage of this topic, there is new research that may shed light on issues of theodicy that Christians can analyze. Snake venoms are complex protein mixtures that are deadly if they wind up in the body of a prey species. They have also been used in the medical field as antivenins for snake bite victims and hold great promise in being used to decrease suffering in areas like cancer research (Holland 2013). One researcher explains; “Our results demonstrate that the evolution of venoms is a really complex process. The venom gland of snakes appears to be a melting pot for evolving new functions for molecules, some of which are retained in venom for killing prey, while others go on to serve new functions in other tissues in the body” (Fitzgerald 2012). A co-author of the same research summarizes: “Many snake venom toxins target the same physiological pathways that doctors would like to target to treat a variety of medical conditions. Understanding how toxins can be tamed into harmless physiological proteins may aid development of cures from venom” (Fitzgerald 2012). Recently Vonk et al. (2013) sequenced the genome of the Indonesian king cobra (Ophiophagus hannah) and compared it with other vertebrate species. The data “. . . confirmed the hypothesis that the individual toxins that make up the venom developed from proteins that originally evolved for unglamorous day-to-day tasks elsewhere in the body. The genes responsible for these proteins have been duplicated many times, allowing the original version to keep doing its job while the copy mutates further and takes on new functions. This allows the proteins taken up by the venom system to multiply and diversify away from their original purposes and contribute to the increasingly complex venomous cocktail, forming lethal suites of chemicals that interact with each other to do more harm to prey” (Marshall 2013). The above research may have ramifications, both in the areas of theodicy and Serpentes diversification since the Fall.
Currently the Burmese python (Python molurus bivittatus) is being used as a model for studying the high diversity found in all snakes. Burmese pythons, and probably other species, can experience rapid physiological changes two to three days after feeding which include; a 44% increase in metabolic rate (higher than any other tetrapod); 35–100% increase in heart, liver, pancreas, small intestine, and kidneys; 160-fold increase in plasma fatty acids and triglyceride content; and a 5-fold increase in microvillus length (Castoe et al. 2011). When digestion is complete these phenotypic and metabolic changes reverse to a state they were prior to feeding. Castoe et al. (2013) found “massive rapid changes in gene expression that coordinate major changes in organ size and function after feeding.” These findings bring more insight into the complexity of chemical pathways, how they affect metabolism and phenotypic plasticity, and may reveal mechanisms for rapid diversification yet to be discovered.
Though they share many traits with other squamates, there are a few traits unique to them and they include: pectoral girdle and forelimbs absent but some may have a “rudimentary” pelvic girdle or hindlimbs in the form of a bony spur, 120 to 500 vertebrae, each neck and trunk vertebra has a pair of ribs making them extremely flexible, in the cranium the supraoccipital is excluded from the margin of the foramen magnum by exoccipitals, flexible ligamentous symphysis between the dentaries allows the right and left jaw to widely spread apart, left lung absent or largely reduced, dominant right lung, ophthalmic branch of the trigeminal nerve is enclosed within the brain case and enters the orbit through the optic foramen (in other squamates the nerve is not enclosed and enters the orbit posteriorly), the left arterial arch is larger than the right (in all other tetrapods it is the reverse), and the absence of ciliary-body muscles in the eye (Pough et al. 2004, pp. 141–142; Vitt and Caldwell 2009, pp. 551–553).
Within the evolutionary naturalist’s paradigm, the origin of snakes is unresolved and their phylogeny is in constant flux. All evolutionists agree that snakes are descendants of lizards because snakes share derived similarities with extent terrestrial varanid lizards and extinct marine mosasaurs (Lee 2005). Therefore, there are two competing evolutionary hypotheses for snake origins. One hypothesis is that snakes are descendants of some ancestral burrowing varanid, in part, because of their unique eye structure (Lee 2005; McDowell 1972). Snake eyes are so different that it is thought that they evolved from vestigial eyes of a burrowing lizard. The other hypothesis is that snakes are descendants of marine lizards related to the mosasaurs (Lee 2005). Lee (2005) attempted to refute the marine lizard hypothesis but after having combined both molecular and morphological data and the inherent naturalistic assumptions, he found that snakes were more closely aligned with marine lizards and could not refute the marine hypothesis.
Fossil Snakes with “Legs”
Adding to the discussion are the fossil “snakes with legs” which are Pachyrhachis problematicus, Haasiophis terrasanctus, and Eupodophis descouensi (Rage and Escuillié 2003). Along with the molecular and morphological data, these have been used to argue that snakes used to have legs, consistent with a lizard origin and nesting within the lizard taxon. From a creationary perspective this hypothesis is possible in that the idea of losing features through natural processes is within the realm of the biblical worldview of Creation and the Fall. Loss of limbs has also been correlated with changes in the expression of some regulatory genes, including Hox gene expression and structure (Castoe et al. 2011). However, the issue of whether these are legs and how they fit into the snake origins narrative is more complicated when viewed in depth. For example, the legs that were identified on the fossils are very small in relation to the size of the body, in some cases only 2 cm (0.7 in), and are nearly comparable with the horny spurs found on pythons (Sarfati 2008). The “leggy snakes” are also found in the same Cenomanian stage (early, late Cretaceous stratum) and restricted to the same Mediterranean region which suggests that they are contemporary with each other (Rage and Escuillié 2003). Complicating matters, there are legless fossil snakes lower in the strata than the leggy ones and both Haasiophis and Pachyrhachis have the “advanced” snake features that enabled them to dislocate their jaws, causing some researchers to conjecture whether some snakes lost legs and then re-evolved them (Hecht 2000).
The bony spurs found on snakes, like pythons and boas, may have an alternative interpretation other than a “rudimentary” pelvic girdle and legs. Today, most snakes do not have these structures, and the snakes that do use them for gripping one another during the mating process.
When considering the above, snake origins can be interpreted in different ways, depending on one’s worldview. As for the competing evolutionary hypotheses, Sarfati (2008) explains; that the “. . . features that are alleged to show common ancestry according to one theory, must really be homoplasies, i.e. convergent evolution of features that arose independently, if the other theory were right. But homology is alleged to be the evidence for evolution (despite many problems) . . .” He goes on to argue that maybe the problem with evolutionary naturalist interpretations to connect snakes with other creatures is because snakes were directly created by God and did not evolve from anything.
What follows is a delineation of the taxa and estimated name and number of kinds, brief descriptions, and average snout to vent length (SVL) or average total length (TL) as ascertained by Vitt and Caldwell (2009) and the Uetz (2013). Massive taxonomic changes continue to be made and updated for Serpentes. This is the most current understanding based on data obtained by the Uetz (2013).
Superfamily Acrochordoidea
File Snake kind
Family Acrochordidae
1 genus Acrochordus—3 species—TL = 90 cm (35 in)

Source: http://en.wikipedia.org/wiki.
Fig. 5. Acrochordus arafurae.
Located in Southeast Asia and northern Australia, these snakes have the following characteristics: small head; loose skin; thick bodies; one left carotid artery; mandible lacking a coronoid bone; girdle and limb elements absent; numerous small and non-overlapping bristle-tip tubercles cover skin and are fully aquatic (Vitt and Caldwell 2009, p. 566). They do not maneuver on land very well and are ovoviviparous, giving birth to live young in the water. Females can also delay conception until environmental conditions are good for giving birth (Houston and Shine 1994). They feed primarily on fish and observations support that when a fish makes contact with the snake’s body, the snake immediately wraps around it, entangles it in the baggy folds of its skin, holds it in place with the bristle-tipped tubercles, and swallows it (Vitt and Caldwell 2009, p. 566). Because of its fully aquatic existence and capability of osmoregulating in hypotonic and hypertonic aquatic environments, it is potentially capable of surviving Flood conditions and are not included on the Ark.
Superfamily Uropeltoidea
4. Pipe Snake Kind
Family Anomochilidae
1 genus Anomochilus—3 species
Cylindrophiidae
1 genus Cylindrophis—10 species—SVL = 50 cm (19 in)

Source: http://en.wikipedia.org/wiki.
Fig. 6. Anomochilus leonardi.
Located in Southeastern Asia, shared characters include short tails; thick bodies; smooth, shiny ventral scales; two common carotid arteries; coronoid bone on mandible; hindlimb “vestiges” appear as cloacal spurs; reduced left lung; tracheal lung absent; and the left and right oviducts are well developed (Vitt and Caldwell 2009, p. 559). These families used to be together in the Cylindrophiidae family, but have since been split into their own families. Though there are minor differences other than average size, they have been kept together as one kind because of their strong cognita.
Uncertain Classification (Incertae sedis) of Superfamily: Pipe-Snake–Like Species
5. False Coral Snake Kind
Family Aniliidae (synonym—Ilysiidae)
Anilius scytale (Monotypic)—TL = 60 cm (23 in)

Source: http://en.wikipedia.org/wiki.
Fig. 7. Anilius scytale.
Some aspects of the taxonomy of this snake, including what superfamily it belongs to, are uncertain, so it has been kept as a separate kind to avoid underestimating the numbers. Morphology is similar to pipe snakes. It has a defensive behavior where it flattens out when danger approaches and lifts the tail off the ground, waving it around as it slithers away or tightens into a ball (Vitt and Caldwell 2009, pp. 258–259).
6. Shield-Tail Snake Kind
Family Uropeltidae
8 genera—51 species—SVL = 25 cm (10 in)
From Sri Lanka and southern India, these snakes share the following characters: cone-shaped heads with keratinized tips and a unique and enlarged scale at the end of the tail (Vitt and Caldwell 2009, p. 560). These snakes are fossorial and when danger approaches they begin burrowing while waving their shielded tail at the attacker (Vitt and Caldwell 2009, p. 560).
Superfamily Pythonoidea
7. Python Kind
Family Pythonidae
9 genera—40 species—TL = 4 m (13 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 8. Python molurus.
Pythons are generally large to very large snakes from sub-Sahara Africa, Southeast Asia and Australia. Characters are similar to other snakes but also include: infrared receptors in the interlabial pits that can detect temperature differences of .05°C, and cloacal spurs (Vitt and Caldwell 2009, p. 563). It has also been reported that at least some species may give birth by parthenogenesis (Booth et al. 2011). Interspecific hybridization has been reported and intergeneric hybrids connect the following genera; Python × Liasis, Morelia × Liasis, Morelia × Python, Aspidites × Python. Through the current hybridization data, at least four of the nine genera make one monobaramin (Banks and Schwaner 1984; Hennigan 2005; Hybridherps 2013).
8. Mesoamerican Python Kind
Family Loxocemidae
Monotypic (Loxocemus bicolor)—SVL = 1 m (3 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 9. Loxocemus bicolor.
Native from southern Mexico to Costa Rica, their biology is not well known and their characters are similar to other pythons. They are kept as a separate kind so numbers are not underestimated.
9. Sunbeam Snake kind
Family Xenopeltidae
1 genus Xenopeltis
2 species—TL = 80 cm (31 in)

Source: http://en.wikipedia.org/wiki.
Fig. 10. Xenopeltis unicolor.
Sunbeam snakes can be found in Southeast Asia and get their name from the glow of their iridescent scales when light hits them (Vitt and Caldwell 2009, p. 562).
Superfamily Booidea
Family Boidae
12 genera—55 species
General characters shared in family Boidae are similar to pythons, but boas are ovoviviparous. Recently it has been reported that boa females can give birth by parthenogenesis (Booth et al. 2011).
10. True Boa Kind
Subfamily Boinae
7 genera—31 species—TL = 4 m (13 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 11. Boa constrictor.
Found in the tropical Americas and islands in the Southwest Pacific, this family includes the giant Anaconda (Eunectes sp.) that may reach lengths of 11.5 m (38 ft) (Vitt and Caldwell 2009, p. 564). Interspecific hybrids of the emerald tree boa (Corallus caninus) and Amazon tree boa (Corallus hortulanus) have been reported amongst snake breeders (Hybridherps 2013).
11. Exiliboa Kind
Subfamily Ungaliophiinae
2 genera—3 species—TL = 45 cm (17 in)

Source: http://reptile-database.reptarium.cz.
Fig. 12. Exiliboa placata.
These species made of the two genera Exiliboa (monotypic) and Ungaliophis used to be included in Boinae but Wilcox et al. (2002), using DNA sequence analysis, have placed them in their own subfamily (Uetz 2013). So kinds are not underestimated, they will be kept separate until further data clarifies their taxonomic status.
12. Sand/Rubber Boa Kind
Subfamily Erycinae
4 genera—16 species—TL = 70 cm (27 in)

Source: http://en.wikipedia.org/wiki.
Fig. 13. Eryx jaculus.
This subfamily of rubber, sand, and rosy boas are semifossorial snakes that can be found in western North America, Central Africa, and Asia (Vitt and Caldwell 2009, p. 564).
Incertae sedis Subfamilies of Boa-Like Snakes
13. Split-Jaw Boa Kind
Family Bolyeridae
2 genera—2 species (1 extinct)—SVL = 1.1 m (3.6 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 14. Casarea dussumieri.
Slender, boa-like, oviparous snakes without cloacal spurs, bolyerids are endemic to Mauritius and Round Island (island north of Mauritius) and are unique because they possess a maxillary that is divided and hinged into front and back structures which produces a split jaw (Vitt and Caldwell 2009, p. 560). The round island burrowing boa (Bolyeria multocarinata) is now considered extinct (IUCN Red List 2013).
14. Dwarf Boa Kind
Family Tropidophiidae
2 genera (Trachyboa and Tropidophis)
25 species—SVL = 60 cm (23 in)

Source: http://reptiledatabase.reptarium.cz.
Fig. 15. Trachyboa boulengeri.
Found in the West Indies, Central America, and South America they were considered a sister taxon to Ungaliophiinae, are primarily snakes of the forest, and are ovoviviparous (Vitt and Caldwell 2009, p. 557).
15. Spine-Jaw Snake Kind
Family Xenophidiidae
1 Genus (Xenophidion)
2 Species—SVL=30 cm (11 in)
Endemic to Malaysia, this genus used to be nested within Tropidophiidae until Lawson, Slowinski, and Burbrink (2004) put them into their own family using cytochrome B analyses and suggested that they are a sister taxon to Bolyeridae (Uetz 2013). They have a unique anterior canine-like tooth on the dentary with the hypothesized function of holding on to small vertebrate prey (Vitt and Caldwell 2009, p. 558).
Superfamily Colubroidea
Recently this group has been taxonomically updated to consist of seven families, 17 subfamilies and greater than 2500 species based on molecular analysis and evolutionary assumptions (Pyron et al. 2011; Uetz 2013). It is a very large group and in great taxonomic flux. Any information discussed below is very tentative. In order to identify the estimated kinds in this complex and diverse taxon, what follows is a breakdown of the families and subfamilies based on molecular analysis.
Family Colubridae
This family consists of seven subfamilies according to the most recent analysis (Chen et al. 2013; Pyron et al. 2011; Pyron, Burbrink, and Wiens 2013). There are venomous species in this family and they differ from other venomous snakes in that they have opisthoglyphous (rearward grooved) teeth that have single grooves along each large tooth. Venom travels down these grooves and is injected in an organism when a bite is delivered. Based on molecular data and much taxonomic uncertainty, kinds will be delineated at the subfamily, but may be lower, and should be considered extremely tentative.
16. King Snake Kind
Subfamily Colubrinae
97 genera—711 species—SVL = 1.5 m (5 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 16. Lampropeltis elapsoides.
This diverse taxon has a worldwide distribution and is connected by several molecular characters. Five genera (Pantherophis, Lampropeltis, Pituophis, Elaphe, and Coelognathus) unite 47 species through intergeneric hybridization and constitute one monobaramin (Hennigan 2005; Hybridherps 2013; Vitt and Caldwell 2009, p. 570). Interspecific hybridization has been reported in Chilomeniscus and Conopsis making two more monobaramins in this subfamily (Hennigan 2005).
17. African Water Snake Kind
Subfamily Grayiinae
1 genus (Grayia, synonym Lycodonomorphus)
4 species—TL = 60 cm (23 in)

Source: http://en.wikipedia.org/wiki.
Fig. 17. Lycodonomorphus rufulus.
18. Dwarf Burrowing Snake Kind
Subfamily Calamariinae
6 genera—87 species—TL = 50 cm (19 in)
19. Hognose Snake Kind
Subfamily Dipsadinae
About 89 genera—742 species
TL = 50 cm (19 in)

Source: http://en.wikipedia.org/wiki.
Fig. 18. Heterodon platirhinos.
20. Mountain Snake Kind
Subfamily Pseudoxenodontinae
2 genera—11 species—TL = 50 cm (19 in)
21. Garter Snake Kind
Subfamily Natricinae
About 31 genera—220 species
SVL = 50 cm (19 in)

Source: http://en.wikipedia.org/wiki.
Fig. 19. Thamnophis elegans terrestris.
Interspecific hybridization has been documented and two monobaramins have been identified; Thamnophis and Nerodia (Hennigan 2005). Once thought to be non-venomous, garter snakes produce a mild neurotoxin and have a posterior tooth.
22. Neck-Band Snake Kind
New Subfamily Scaphiodontophiinae
1 genus (Scaphiodontophis)—2 species
(Chen et al. 2013; Pyron et al. 2011; Pyron, Burbrink, and Wiens 2013)
Taxonomy still in flux—TL = 40 cm (16 in)

Photograph: Jario Maldonado.
Fig. 20. Scaphiodontophis annulatus.
Family Lamprophiidae
This family consists of seven subfamilies and 301 species. Because so much is unknown, the kind will be delineated at the subfamily, based on molecular analysis, and should be considered very tentative.
23. African Rear-Fanged Snake Kind
Subfamily Aparallactinae
10 genera—50 species—TL = 40 cm (16 in)
24. Mole Viper Kind
Subfamily Atractaspidinae
2 genera—22 species—TL = 50 cm (19 in)

Source: http://en.wikipedia.org/wiki.
Fig. 21. Atractaspis engaddensis.
25. African House Snake Kind
Subfamily Lamprophiinae
12 genera—68 species—TL = 65 cm (26 in)

Source: http://en.wikipedia.org/wiki.
Fig. 22. Lamprophis fuliginosus.
26. African Beaked/Sand Snake Kind
Subfamily Psammophiinae
7 genera—49 species—TL = 50 cm (19 in)

Source: http://en.wikipedia.org/wiki.
Fig. 23. Psammophis trinasalis.
27. African Shovelsnout Snake Kind
Subfamily Prosymninae
1 genus—16 species—TL = 35 cm (14 in)

Source: http://reptiledatabase.reptarium.cz. Photograph: Kate Jackson.
Fig. 24. Prosymna ambigua.
28. African Mole/Keeled Snake Kind
Subfamily Pseudaspidinae
2 genera—2 species—TL = 1.2 m (4 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 25. Pseudaspis cana.
29. Malagasy Leaf Snake Kind
Subfamily Pseudoxyrhophiinae
22 genera—88 species—TL = 60 cm (23 in)
(Many genera are found in Madagascar;
others are found in Africa and parts of the Middle East)

Source: http://en.wikipedia.org/wiki.
Fig. 26. Leioheterodon madagascariensis.
Superfamily Elapoidea
Family Elapidae
2 Subfamilies—354 species
This family consists of venomous snakes and include the cobras, kraits, sea snakes, death adders, coral snakes, and allies. A common character they possess is proteroglyphous (forward grooved) fangs which are fixed (non-hinged), forward placed, and with a closed canal in which venom is dispensed (in contrast to rear “grooved” teeth in colubrids). Spitting cobras (Naja sp.) have specially designed venom apparati that enable them to fire venom at a distance and accurately hit an organism in the eyes. Some members are fully marine and ancestors could survive the Flood, therefore a few will not be counted as Ark kinds. Their morphology is similar to other snakes except for the fact that the tracheal lung is commonly present in marine species, but is absent in terrestrial ones (Vitt and Caldwell 2009, p. 574). There is some disagreement as to the taxonomy of this family. Kelly et al. (2009) do infer equivalent groups with Pyron et al. (2011). The former splits superfamily Elapoidea into five families: Atractaspididae (including Atractaspidinae and Aparallactinae), Lamprophiidae, Prosymnidae, Psammophiidae, Pseudaspididae, Pseudoxyrhophiidae (including Pseudoxyrhophiinae and Amplorhininae—Uetz 2013). Since relationships remain unresolved, the kind will be tentatively delineated at the subfamily or lower.
Subfamily Hydrophiinae
This taxon continues to be confusing and is made up of both terrestrial and aquatic taxa. The aquatic group is interpreted, by some, as consisting of two marine clades that are well designed for living in water (Vitt and Caldwell 2009, p. 575). The terrestrial members consist of semi-aquatic, surface feeding, semi-arboreal, and semi-fossorial species (Vitt and Caldwell 2009, p. 575). Based on molecular data and design the tentative kinds are delineated below.
True Sea Snake Kind
8 genera—62 species:
Aipysurus (8 species),
Emydocephalus (3 species),
Ephalophis (1 species),
Hydrelaps (1 species),
Hydrophis (45 species),
Kolpophis (1 species),
Parahydrophis (2 species),
Thalassophis (1 species)—SVL = 1.2 m (3.9 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 27. Aipysurus laevis.
These marine snakes are totally aquatic and have shared characters that include: laterally compressed body, ovoviviparity (babies are born in the water), highly venomous, laterally flattened paddle-like tail, no ventral scales, incapable of terrestrial locomotion, and piscivorous (Uetz 2013 ; Vitt and Caldwell 2009, p. 576). Genera and species were identified in order to break down the subfamily into kinds based on morphology and behavioral design. Because they could have survived the Flood, they are not counted as an Ark kind.
30. Sea Krait Kind
1 genus (Laticauda)
7 species—SVL = 90 cm (35 in)

Source: http://en.wikipedia.org/wiki.
Fig. 28. Laticauda colubrina.
Sea kraits are designed to live on both land and water. Characters include reduced ventral scales, regularly come on land, good terrestrial locomotion, oviparity where they must lay eggs on land, some use the shore to bask and digest food, more cylindrical morphology, and venomous (Vitt and Caldwell 2009, p 576). So as to not underestimate kinds, they are included on the Ark.
31. Cobra Kind
Subfamily Elapinae
About 46 genera and 285 species—TL = 3.5 m (11.5 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 29. Naja naja.
Much is still very confusing about the taxonomy of this group. Until further research clarifies relationships, the kind is here identified at the subfamily. There is a lot of variability in this group and they can be found in Eurasia, Africa, and the Americas (Vitt and Caldwell 2009, p. 575). Well known members are the cobras, coral snakes, and death adders—all of which are venomous.
32. Australo-Asian Water Snake Kind
Family Homalopsidae
12 genera—52 species—TL = 80 cm (31 in)

Source: http://en.wikipedia.org/wiki.
Fig. 30. Cerberus rynchops.
Formerly this taxon was subfamily Homalopsinae in Colubridae. They share the following characters: valvular, crescent dorsal nostrils; dorsally oriented eyes; ovoviviparity; last two or three maxillary teeth grooved with well-developed venom; inhabit shallow water (with the exception of Brachyorrhos and Calamophis that are semifossorial; terrestrial, and sometimes aquatic species). Brachyorrhos has been inferred to be a basal taxon of Homalopsidae (Murphy 2012; Murphy et al. 2012; Vitt and Caldwell 2009, p. 571). The tentacle snake has been separated from this group as a separate kind because of its unusual traits.
33. The Tentacle Snake Kind
Family Homalopsidae
1 genus (Erpeton)
1 species (tentaculatum)—TL = 70 cm (27 in)

Source: http://en.wikipedia.org/wiki.
Fig. 31. Erpeton tentaculatum.
This is an unusual water snake from Thailand, Cambodia, and Vietnam that is the only snake to have two tentacles on the front of its head used as mechanosensors for detecting prey (Uetz 2013). They live all their lives in water bodies that include fresh, brackish, and saltwater and can be underwater for many minutes at a time. If they are not a post Flood phenomenon, they have the capability of osmoregulating in various aquatic environments, could potentially survive the Flood, and are therefore not included in the Ark count.
34. Slug Snake Kind
Family Pareatidae
3 genera—18 species—TL = 62 cm (24 in)

Source: http://en.wikipedia.org/wiki.
Fig. 32. Pareas margaritophorus.
From Southeast Asia, shared characters include blunt snout, no mental groove, and no teeth on anterior part of the maxillary (Vitt and Caldwell 2009, p. 572).
Family Viperidae
The viper family currently consists of three subfamilies and 320 species and found worldwide except for Papuaustralia and oceanic islands (Uetz 2013; Vitt and Caldwell 2009, p. 566). Common characters include long, rotating solenoglyphous (pipe grooved) fangs that fold up against roof of mouth; venom travels through a closed canal (pipe groove) in teeth; most have robust bodies and triangular heads; other body morphology similar as other snakes except that in crotalines infrared receptors capable of detecting .05°C temperature differences occur in loreal pits on the face (which is where the term “pit viper” comes from), and in other taxa the heat receptors are located beneath scale surfaces (Vitt and Caldwell 2009, p. 567). For the purposes of this analysis, rattlesnakes have been identified as a separate kind within Crotalinae because the rattle is a unique synapomorphy common to both rattlesnake genera.
35. Fea Viper kind
Subfamily Azemiopinae
1 genus (Azemiops)—2 species—TL = 66 cm (26 in)

Source: http://en.wikipedia.org/wiki.
Fig. 33. Azemiops feae.
Fea vipers are endemic from South-Central China to Burma and Vietnam, and tend to be found in moist montane forests (Vitt and Caldwell 2009, p. 567).
36. Rattlesnake Kind
Subfamily Crotalinae
2 genera—38 species—TL = 95 cm (37 in)

Source: http://en.wikipedia.org/wiki.
Fig. 34. Crotalus cerastes.
Endemic to Southwest and southern Asia and the Americas, these snakes have well developed infrared sensors in the loreal pit (Vitt and Caldwell 2009, p. 568). Intergeneric hybridization has been reported for two genera of rattlesnakes (Sistrurus/Crotalus) encompassing 38 species; this constitutes a monobaramin within Crotalinae and possibly a holobaramin because the rattle is unique to this group (Hennigan 2005; Uetz 2013).
37. Copperhead/Moccasin Kind
Subfamily Crotalinae
18 genera—188 species—TL = 80 cm (31 in)
Interspecific hybridization was recorded in Agkistrodon (six species including cantils, copperheads, and moccasins) constituting a monobaramin (Hennigan 2005). The southern bushmaster (Lachesis muta) is the largest crotaline and can reach lengths of 3.75 m (12.5 ft) (Vitt and Caldwell 2009, p. 568).
38. Adder Kind
Subfamily Viperinae
13 genera—91 species—TL = 1 m (3 ft)

Source: http://en.wikipedia.org/wiki.
Fig. 35. Dabioa russelii.
Endemic to Africa and Eurasia, viperines do not have loreal pits. Instead, infrared sensors are located under scale surfaces (Vitt and Caldwell 2009, p. 569). Interspecific hybridization has been reported in Bitis, which encompasses 17 species into one monobaramin (Hennigan 2005; Uetz 2013).
39. Odd-Scaled Snake Kind
Family Xenodermatidae
5 genera—17 species—TL = 50 cm (10 in)

Source: http://en.wikipedia.org/wiki.
Fig. 36. Achalinus formosanus.
Formerly a colubrid subfamily, xenodermatids have been reclassified into their own family. They have a disjunct distribution in Asia (Uetz 2013). Shared characters include an optic nerve exiting through small orbits, a unique morphology where the ophthalmic nerve exits through a foramen in the parietal, and greater than 20 maxillary teeth (Vitt and Caldwell 2009, p. 572). For this analysis the kind is delineated at the family because of their unique morphological and molecular characters and because there is still much that is not known.
Superfamily Typhlopoidea
(synonym Scolecophidia)
Generally known as the blind snakes, this superfamily is currently made up of five families and two subfamilies. It is quite possible that these taxa may comprise a holobaramin. But, so that numbers are not underestimated, approximate kinds are inferred below.
40. Dawn Blind Snake Kind
Family Anomalepididae
4 genera—18 species—TL = 23 cm (9 in)

Source: http://reptiledatabase.reptarium.cz.
Fig. 37. Typhlophis squamosus.
Endemic to Central and South America in disjunct populations, their morphology is similar to other snakes, particularly other blind snakes. It is possible that they are part of a larger blind snake holobaramin, but they are included until a better understanding of their taxonomic relationships is elucidated and so that numbers are not underestimated (Vitt and Caldwell 2009, p. 554).
41. Worm Snake Kind
Family Gerrhopilidae
1 genus (Gerrhopilus: synonym Typhlops) 15 species—TL = 14 cm (5.5 in)
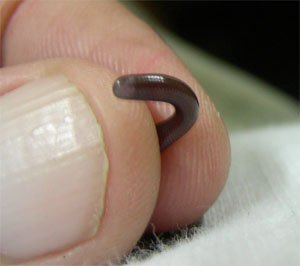
Source: http://en.wikipedia.org/wiki.
Fig. 38. Typhlops.
42. Blind Snake Kind
Typhlopidae
8 genera—254 species—TL = 55 cm (21 in)

Source: http://en.wikipedia.org/wiki.
Fig. 39. Ramphotyphlops braminus.
Family Leptotyphlopidae
This taxon consists of two subfamilies and the kinds will be delineated at the subfamily, based on current molecular analysis, until there is a better understanding of their taxonomic status.
43. Thread Snake Kind
Subfamily Leptotyphlopinae
4 genera—52 species—SVL = 25 cm (10 in)

Source: http://en.wikipedia.org/wiki.
Fig. 40. Leptotyphlops humilis.
44. Slender Blind Snake Kind
Subfamily Epictinae
6 genera—50 species—SVL = 25 cm (10 in)

Source: http://en.wikipedia.org/wiki.
Fig. 41. Trilepida macrolepis.
45. Malagasy Blind snake Kind
Family Xenotyphlopidae
1 genus (Xenotyphlops) 2 species—SVL = 25 cm (10 in)
Conclusions
The challenge of using a biblical worldview to determine Ark kinds is distinguishing if similar characters are products of a common Designer or common ancestry within a kind, and when discontinuities indicate a separate kind. Also, there are over-simplified beliefs that snakes don’t have legs because God cursed them to eat the dust of the ground. There are many creatures that are legless and slither and certainly there is more to the serpent in the Garden than a mere animal. The focus of both Genesis 3 and Revelation 20 seems to require that we look deeper into what is behind the serpent in the Garden rather than have some simplistic explanation for why snakes don’t have legs.
The recent findings of Vonk et al. (2013) and Castoe et al. (2013) suggest systems that were working for good before the Fall can rapidly change into systems that brought harm. The genetic mechanisms involved in these types of rapid changes are of interest to creation biologists in order to better understand how organisms could diversify rapidly and how normal protein synthesis for metabolic processes, typical for organism survival and function, could genetically change in order to meet the demands of environmental stresses in a fallen world.
The fact that naturalists have a hard time connecting all reptiles to one or a few common ancestors suggests that they are not all related to one another, but instead were created separately according to their kind. Similar biochemistry across diverse taxa can also be interpreted as a function of having a common Designer, rather than being related by universal common descent. The fact that tuataras, amphisbaenas, and snakes are marvelously designed and are well endowed with structures that surpass human technology is consistent with a wise and all powerful engineer behind their origins.
After carefully reviewing the molecular and hybridization data, but recognizing that there is much that we do not know or even understand, it is suggested that one extant tuatara, two extant amphisbaena, and 41 extant snake kinds may have been brought on the Ark. Since their exit from the Ark, they have diversified into the plethora of species we marvel at today. No matter how many were included on the Ark, reptile diversity and persistence are a reminder of an all-powerful Creator who is both just and merciful.
References
Banks, C., and T. D. Schwaner. 1984. Two cases of interspecific hybridization among captive Australian boid snakes [Python spilotes, Python amethistinus, Liasis mackloti.] Zoo Biology 3, no. 3:221–227.
Booth, W., D. H. Johnson, S. Moore, C. Schal, and E. LO. Vargo. 2011. Evidence for viable, non-clonal but fatherless Boa constrictors. Biology Letters 7, no. 2:253–256. Retrieved from http://rsbl.royalsocietypublishing.org/content/7/2/253.full.pdf+html on December 7, 2013.
Brophy, T. R., and P. A. Kramer. 2007. Preliminary results from a baraminological analysis of the mole salamanders (Caudata: Ambystomatidae). Occasional Papers of the BSG 10:10–24.
Castoe, T. A., A. P. J. de Koning, K. T. Hall, K. D.Yokoyama, W. Gu, E. N. Smith, C. Feschotte, et al. 2011. Sequencing the genome of the Burmese python (Python molurus bivittatus) as a model for studying extreme adaptations in snakes. Genome Biology 12, no. 7:406.
Castoe, T. A., A. P. J. de Koning, K. T. Hall, D. C. Card, D. R. Schield, M. K. Fujita, R. P. Ruggiero, et al. 2013. The Burmese python genome reveals the molecular basis for extreme adaptation in snakes. PNAS abstract retrieved from http://www.pnas.org/content/early/2013/11/27/1314475110 on December 8, 2013.
Chen, X., S. Huang, P. Guo, G. R. Colli, A. Nieto-Montes de Oca, L. J. Vitt, R. A. Pyron, and F. T. Burbrink. 2013. Understanding the formation of ancient intertropical disjunct distributions using Asian and Neotropical hinged-teeth snakes (Sibynophis and Scaphiodontophis: Serpentes: Colubridae). Molecular Phylogenetics and Evolution 66, no. 1: 254–261.
Fitzgerald, K. 2012. Snake venom could unlock drug discoveries for cancer, diabetes. Medical News Today, September 20, 2012. Retrieved from http://www.medicalnewstoday.com/articles/250455.php on December 13, 2012.
Handwerk, B. 2010. Evolution in action: Lizard moving from eggs to live birth. National Geographic Daily News, September 1, 2010. Retrieved from http://news.nationalgeographic.com/news/2010/09/100901-science-animals-evolution-australia-lizard-skink-live-birth-eggs/ on July 18, 2013.
Hay, J. M., S. D. Sarre, D. M. Lambert, F. D. Allendorf, and C. H. Daugherty. 2010. Genetic diversity and taxonomy: A reassessment of species designation in tuatara (Sphenodon: Reptilia). Conservation Genetics 11, no. 3:1063–1081.
Hecht, J. 2000. Prehistoric pins. New Scientist 165. no. 2231:12.
Helder, M. 1991. Tantalizing tuatara. Creation 13, no. 3:24–27. Retrieved from http://creation.com/tantalizing-tuatara on December 12, 2013.
Hennigan, T. 1998. Snakes alive! There’s design in the Curse. Creation 20, no. 4:40–42. Retrieved from http://creation.com/snakes-alive on December 12, 2013.
Hennigan, T. 2005. An initial investigation into the baraminology of snakes: Order—Squamata, Suborder Serpentes. Creation Research Society Quarterly 42, no. 3:153–160.
Hennigan, T. 2013a. An initial estimate toward identifying and numbering amphibian kinds within the orders caudata and gymnophiona. Answers Research Journal 6:17–34. Retrieved from http://www.answersingenesis.org/articles/arj/v6/n1/amphibian-kinds.
Hennigan, T. 2013b. An initial estimate toward identifying and numbering the frog kinds on the Ark: Order anura. Answers Research Journal 6:335–365. Retrieved from http://www.answersingenesis.org/articles/arj/v6/n1/frog-kinds-on-ark.
Hennigan, T. 2014. An initial estimate toward identifying and numbering the Ark turtle and crocodile kinds. Answers Research Journal 7:1–10. Retrieved from http://www. answersingenesis.org/articles/arj/v7/n1/turtle-crocodile-kinds.
Holland, J. S. 2013. The bite that heals. National Geographic 223, no. 2. Retrieved from http://ngm.nationalgeographic.com/2013/02/125-venom/holland-text on December 12, 2013.
Houston, D., and R. Shine. 1994. Low growth rates and delayed maturation in Arafura file snakes (Serpentes: Acrochordidae) in tropical Australia. Copeia 3:726–731.
Hybridherps. 2013. Retrieved from http://www.hybridherps.com/descriptions/ on December 12, 2013.
IUCN. 2013. The IUCN Red List of Threatened Species. Version 2013.2. International Union for the Conservation of Nature. Retrieved from http://www.iucnredlist.org on December 12, 2013.
Jensen, J. B., C. D. Camp, W. Gibbons, and M. J. Elliot (eds). 2008. Amphibians and reptiles of Georgia. Athens, Georgia: University of Georgia Press.
Kelly, C. M. R., N. P. Barker, M. H. Villet, and D. G. Broadley. 2009. Phylogeny, biogeography, and classification of the snake superfamily Elapoidea: a rapid radiation in the late Eocene. Cladistics 25, no. 1:38–63.
Kiwi Conservation Club. 2013. Tuatara. Retrieved from http://kcc.org.nz/tuatara on December 12, 2013.
Lawson, R., J. B. Slowinski, and F. T. Burbrink. 2004. A molecular approach to discerning the phylogenetic placement of the enigmatic snake Xenophidion schaeferi among the Alethinophidia. Journal of Zoology 263:285–294.
Lee, M. S. Y. 2005. Molecular evidence and marine snake origins. Biology Letters 1, no. 2:227–230. Retrieved from http://rsbl.royalsocietypublishing.org/content/1/2/227.full.pdf+html on July 20, 2013.
Lightner, J. K. 2008. Life: designed by God to adapt. Retrieved from http://www.answersingenesis.org/articles/aid/v3/n1/life-designed-to-adapt on December 12, 2013.
Lightner, J. K. 2013. An initial estimate of avian Ark kinds. Answers Research Journal 6:409–466. Retrieved from http://www.answersingenesis.org/articles/arj/v6/n1/avian-ark-kinds.
Lightner, J. K., T. Hennigan, and G. Purdom. 2011. Determining the Ark kinds. Answers Research Journal 4:195–201. Retrieved from http://www.answersingenesis.org/articles/arj/v4/n1/ark-kinds-flood-baraminology-cognitum.
Marshall. T. 2013. The rapid evolution of cobra venom. Phys. Org. Retrieved from http://phys.org/news/2013-12-rapid-evolution-cobra-venom.html on December 12, 2013.
McDowell, S. 1972. The evolution of the tongue of snakes, and its bearing on snake origins. BMC Evolutionary Biology 6:191–273.
Murphy, J. C. 2012. Synonymised and forgotten, the bird’s head stout-tailed snakes, Calamophis Meyer (Squamata: Serpentes: Homalopsidae). The Raffles Bulletin of Zoology 60, no. 2:515–523.
Murphy, J. C., Mumpuni, R. de Lang, D. J. Gower, and K. L. Sanders. 2012. The moluccan short-tailed snakes of the genus Brachyorrhos Kuhl (Squamata: Serpentes: Homalopsidae), and the status of Calamophis Meyer. The Raffles Bulletin of Zoology 60, no. 2:501–514.
Pough, F. H., R. M. Andrews, J. E. Cadle, M. L. Crump, A. H. Savitsky, and K. D. Wells. 2004. Herpetology 3rd ed. Upper Saddle River, New Jersey: Pearson Prentice Hall.
Pyron, R. A., F. T. Burbrink, G. R. Colli, A. N. Montes de Oca, L. J. Vitt, C. A. Kuczynski, and J. J. Wiens. 2011. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58, no. 2:329–342.
Pyron, R. A., F. T. Burbrink, and J. J. Wiens. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13:93.
Rage J-C., and F. Escuillié. 2003. The Cenomanian: Stage of hindlimbed snakes. Notebooks on Geology. Retrieved from http://paleopolis.rediris.es/cg/CG2003_A01_JCR-FE/index_uk.html on July 20, 2013.
Sanders, R. W., and K. P. Wise. 2003. The cognitum: A perception-dependent concept needed in baraminology. In Proceedings of the Fifth International Conference on Creationism, ed. R. L. Ivey, Jr., pp. 445–455. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Sarfati, J. 2008. Another leggy snake: What should creationists think? Retrieved from http://creation.com/another-leggy-snake on December 12, 2013.
Turner, K. J. 2009. The kind-ness of God: A theological reflection of Mîn, “kind.” In CORE Issues in Creation no. 5, ed. T. C. Wood and P. A. Garner, pp. 31–64. Eugene, Oregon: Wipf and Stock.
Uetz, P. (ed). Reptile Database. Retrieved from http://www.reptile-database.org/db-info/taxa.html on December 10, 2013.
Vitt, L. J., and J. P. Caldwell. 2009. Herpetology: An introductory biology of amphibians and reptiles. 3rd ed. Burlington, Massachusetts: Academic Press.
Vonk, F. J., N. R. Casewell, C. V. Henkel, A. M. Heimberg, H. J. Jansen, R. J. R. McCleary, H. M. E. Kerkkamp, et al. 2013. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. PNAS Abstract retrieved from http://www.pnas.org/content/early/2013/11/27/1314702110 on December 8, 2013.
Wilcox, T. P., D. J. Zwickl, T. A. Heath, and D. M. Hillis. 2002. Phylogenetic relationships of the dwarf boas and a comparison of Bayesian and bootstrap measures of phylogenetic support. Molecular Phylogenetics and Evolution 25, no. 2:361–371.
Williams, P. J. 1997. What does min mean? CEN Tech J. 11, no. 3:344–352.
Wilson, G. 2010. Classic multidimensional scaling isn’t the sine qua non of baraminology. Retrieved from http://www.answersingenesis.org/articles/aid/v5/n1/cmds-baraminology on December 7, 2013.
Wise, K. P. 1992. Practical baraminology. Creation Ex Nihilo Technical Journal 6, no. 2:122–137.
Wood, T. C. 2003. Mediated design. Impact #363. Institute for Creation Research: El Cajon, California. Retrieved from http://www.icr.org/i/pdf/imp/imp-363.pdf on July 18, 2013.
Wood, T. C., K. P. Wise, R. Sanders, and N. Doran. 2003. A refined baramin concept. Occasional Papers of the BSG 3:1–14.
Wood, T. C. 2006a. The current status of baraminology. Creation Research Society Quarterly 43, no. 3:149–158.
Wood, T. C. 2006b. Statistical baraminology workbook. Unpublished workbook presented at workshop during the BSG conference June 13, 2007, Liberty University, Lynchburg, Virginia.
Woodmorappe, J. 1996. Noah’s Ark: A feasibility study. Santee, California: Institute for Creation Research.














