Research conducted by Answers in Genesis staff scientists or sponsored by Answers in Genesis is funded solely by supporters’ donations.
Abstract
U-Pb radioisotope dating is now the absolute dating method of first choice among geochronologists, especially using the mineral zircon. A variety of analytical instruments have also now been developed using different micro-sampling techniques coupled with mass spectrometers, thus enabling wide usage of U-Pb radioisotope dating. However, problems remain in the interpretation of the measured Pb isotopic ratios to transform them into ages. Among them is the presence of non-radiogenic Pb of unknown composition, often referred to as common or initial Pb. There is also primordial Pb that the earth acquired when it formed, its isotopic composition determined as that of troilite in the Canyon Diablo iron meteorite. Subsequently new crustal rocks formed via partial melts from the mantle. U decay in those rocks added daughter Pb isotopes to the common or initial Pb isotopes in them, inherited from the rock’s sources. So the Pb isotope ratios measured in these rocks today must be interpreted before their U-Pb ages can be calculated. Various methods have been devised to determine this initial or common Pb, but all involve making unprovable assumptions. Zircon does incorporate initial Pb when it crystallizes. The amount of 204Pb cannot be measured independently and accurately. It cannot be demonstrated that the initial Pb only consisted of 204Pb atoms. It cannot be proven that the Pb in apparently cogenetic U- or Th-free minerals is only initial Pb, and that it is identical to the initial Pb in the mineral being dated. Nor can the measured 206Pb, 207Pb, and 208Pb isotope ratios be used to somehow decide what proportions of them are the initial Pb without recourse to unprovable assumptions about the mineral or rock’s history or their interpreted U-Th-Pb ages within an assumed deep time history. Nevertheless, the ultimate foundation of this U-Pb dating methodology is the assumption that the earth formed from the solar nebula. However, from a biblical perspective the earth was created by God on Day 1 of the Creation Week before the sun and the rest of the solar system were created on Day 4, all only about 6000 or so years ago. Yet the earth would still have had an initial (created) Pb isotopic endowment. Once radioactive decay of U and Th started after creation, daughter Pb isotopes were added inside the earth. Then catastrophic plate tectonics during the Flood stirred the mantle and via partial melting added new rocks to the crust. These new rocks rapidly accumulated more Pb isotopes due to the concurrent accelerated radioactive decay of U and Th in them during the Flood. Thus, without being able to unequivocally distinguish the daughter Pb atoms produced by in situ U and Th decay from the initial Pb atoms in a mineral or rock, it is impossible to determine their absolute U-Pb ages. All the unprovable assumptions ultimately depend on an assumed deep time history. Its rejection is recognized as fatal to the earth’s claimed age of billions of years. There is thus no impediment to accepting and using the Bible’s account of Creation and the Flood as a reliable framework for unravelling the history of the earth and the Pb isotopes found in its minerals and rocks.
Keywords: radioisotope dating, 238U, 235U, 206Pb, 207Pb, uranium-lead dating, lead-lead dating, concordia, discordia, Pb-Pb isochrons, common Pb, initial Pb, primordial Pb, 204Pb, common Pb dating, zircon, uncertainties, mass spectrometers, assumptions, geochemical/isotopic reservoirs, Creation Week, Flood
Introduction
Radioisotope dating of minerals, rocks and meteorites is perhaps the most potent claimed proof for the supposed old age of the earth and the solar system. The absolute ages provided by the radioisotope dating methods provide an apparent aura of certainty to the claimed millions and billions of years for formation of the earth’s rocks. Many in both the scientific community and the general public around the world thus remain convinced of the earth’s claimed great antiquity.
The decay of 238U and 235U to 206Pb and 207Pb, respectively, forms the basis for one of the oldest methods of geochronology (Dickin 2005; Faure and Mensing 2005). While the earliest studies focused on uraninite (an uncommon mineral in igneous rocks), there has been intensive and continuous effort over the past five decades in U-Pb dating of more-commonly occurring trace minerals. Zircon (ZrSiO4) in particular has been the focus of thousands of geochronological studies, because of its ubiquity in felsic igneous rocks and its claimed extreme resistance to isotopic resetting (Begemann et al. 2001).
However, accurate radioisotopic age determinations require that the decay constants or half-lives of the respective parent radionuclides be accurately known and constant in time. Ideally, the uncertainty of the decay constants should be negligible compared to, or at least be commensurate with, the analytical uncertainties of the mass spectrometer measurements entering the radioisotope age calculations (Begemann et al. 2001). Clearly, based on the ongoing discussion in the conventional literature this is still not the case at present. The stunning improvements in the performance of mass spectrometers during the past four or so decades, starting with the landmark paper by Wasserburg et al. (1969), have not been accompanied by any comparable improvement in the accuracy of the decay constants (Begemann et al. 2001; Steiger and Jäger 1977), in spite of ongoing attempts (Miller 2012). The uncertainties associated with direct half-life determinations are, in most cases, still at the 1% level, which is still significantly better than any radioisotope method for determining the ages of rock formations. However, even uncertainties of only 1% in the half-lives lead to very significant discrepancies in the derived radioisotope ages. The recognition of an urgent need to improve the situation is not new (for example, Min et al. 2000; Renne, Kamer, and Ludwig 1998). It continues to be mentioned, at one time or another, by every group active in geo- or cosmochronology (Boehnke and Harrison 2014; Schmitz 2012).
From a creationist perspective, the 1997–2005 RATE (Radioisotopes and the Age of The Earth) project successfully made progress in documenting some of the pitfalls in the radioisotope dating methods, and especially in demonstrating that radioisotope decay rates may not have always been constant at today’s measured rates (Vardiman, Snelling, and Chaffin 2000, 2005). Yet much research effort remains to be done to make further inroads into not only uncovering the flaws intrinsic to these long-age dating methods, but towards a thorough understanding of radioisotopes and their decay during the earth’s history within a biblical creationist framework.
One crucial area the RATE project did not touch on was the issue of how reliable are the determinations of the radioisotope decay rates, which are so crucial for calibrating these dating “clocks.” However, in a recent series of papers, Snelling (2014a, b, 2015a, b, 2016, 2017) reviewed how the half-lives of the parent radioisotopes used in long-age geological dating have been determined and collated all the determinations of them reported in the literature to discuss the accuracy of their currently accepted values. He documented the methodology behind and history of determining the decay constants and half-lives of the parent radioisotopes 87Rb, 176Lu, 187Re, 147Sm, 40K, 238U, and 235U which are used as the basis for the Rb-Sr, Lu-Hf, Re-Os, Sm-Nd, K-Ar, Ar-Ar, U-Pb, and Pb-Pb long-age dating methods respectively. He showed that there is still some uncertainty in what the values for these measures of the 87Rb, 176Lu, 40K, and 235U decay rates should be, in contrast to the apparent agreement on the 187Re, 147Sm, and 238U decay rates. This uncertainty is especially prominent in determinations of the 176Lu decay rate by physical direct counting experiments. Furthermore, the determined values of the 87Rb decay rate differ when Rb-Sr ages are calibrated against the U-Pb ages of either the same terrestrial minerals and rocks or the same meteorites and lunar rocks. Ironically it is the slow decay rates of isotopes such as 87Rb, 176Lu, 187Re, and 147Sm used for deep time dating that makes precise measurements of their decay rates so difficult. Thus, it could be argued that direct measurements of their decay rates should be the only acceptable experimental evidence, especially because measurements which are calibrated against other radioisotope systems are already biased by the currently accepted methodology employed by the secular community in their rock dating methods.
Ultimately, the 87Rb, 176Lu, 187Re, 147Sm, and 40K decay half-lives have all been calibrated against the U-Pb radioisotope systems. This is the case even for the 147Sm decay half-life whose accepted value has not changed since it was calibrated against the U-Pb dating of two meteorites in the 1970s, in spite of the fact that more recent thorough physical direct counting experiments suggest a higher value. However, confidence in U-Pb radioisotope dating as the “gold standard” is very questionable, as there are now known small measured variations in the 238U/235U ratio that is critical to that method (Brennecka and Wadhwa 2012; Goldmann et al. 2015; Hiess et al 2012; Tissot and Dauphas 2015), as well as uncertainties as to the 238U and 235U decay rate values (Boehnke and Harrison 2014; Mattinson 2010; Schoene et al. 2006; Schön, Winkler, and Kutschera 2004; Snelling 2017; Villa et al. 2016). It is to be expected that every long-lived radioactive isotope is likely to show similar variation and uncertainty in half-life measurements because these are difficult measurements to make. However, even small variations and uncertainties in the half-life values result in large variations and uncertainties in the calculated ages for rocks and minerals, and the question remains as to whether the half-life values for each long-lived parent radioisotope are independently determined.
Nevertheless, accurate radioisotope age determinations not only depend on accurate determinations of the decay constants or half-lives of the respective parent radioisotopes, but on the reliability of the other two assumptions these supposed absolute dating methods rely on. Those assumptions are the starting conditions and no contamination of closed systems. Both assumptions are unprovable. Yet they can supposedly be circumvented somewhat via the isochron technique, because it is claimed to be independent of the starting conditions and sensitive to revealing any contamination, which is still significantly better than any of the model radioisotope age methods for determining the ages of rock formations. Data points that do not fit on the isochron are simply ignored because their values are regarded as due to contamination. That this is common practice is illustrated with numerous examples cited from the literature by Faure and Mensing (2005) and Dickin (2005). On the other hand, it could be argued that this discarding of data points which do not fit the isochron is arbitrary and therefore is not good science, because it is merely assumed the “aberrant” values are due to contamination rather than that being proven to be so. Indeed, in order to discard such outliers in any data set, one must establish a reason for discarding those data points which cannot be reasonably questioned.
Undoubtedly the U-Pb and Pb-Pb radioisotope dating methods are now the cornerstone in current geochronology studies. Thus it is imperative every aspect of the methodology used in these methods be carefully examined to investigate whether the age results obtained by them are really as accurate and absolute as portrayed in the geological literature. Therefore, it is highly significant that Amelin et al. (2009) listed the potential problems which cause possible inaccuracies in obtaining reliable U-Pb and Pb-Pb ages. These are:
- presence of non-radiogenic Pb of unknown isotopic composition;
- deviations from closed system evolution (gain or loss of U, loss of intermediate daughters such as the inert gas Rn, and loss of Pb);
- misidentification of the processes that start or reset the isotopic clocks;
- analytical problems (fractionation, instrument specific, etc.) and blank subtraction;
- fractionation of radiogenic Pb isotopes induced by leaching via alpha recoil tracks because of that damage to the host minerals’ crystalline structures;
- variations in the 238U/235U ratio;
- uncertainties in the half-lives of 238U and 235U; and
- deviations of the 234U/238U ratio from secular equilibrium.
Of these eight potential problems, Amelin et al. (2009) admitted that the first five are important and common, whereas the last three they considered insignificant or unlikely. But recent research has even found that these last three problems are more critical than they estimated, not least the variations in the 238U/235U ratio (Goldmann et al. 2015; Tissot and Dauphas 2015), and the uncertainties in the half lives of 238U and 235U (Boehnke and Harrison 2014; Snelling 2017). Thus, it is to each of these potential problems we now turn. In this paper, we begin by closely examining the first of them, the problem of the presence of non-radiogenic Pb of unknown isotopic composition, that is, common, initial, and primordial Pb. But before that, there is a need to go over some important background informational issues germane to the subsequent focus on the issue of common, initial and primordial Pb.
Uranium and Lead Geochemistry
Uranium is element 92 (Z = 92) and a member of the actinide series in which the 5f orbitals are progressively filled with electrons. It occurs naturally in the tetravalent oxidation state U4+ with an ionic radius of 1.05 Å. But under oxidizing conditions it forms the uranyl ion (UO22+) in which U has a valence of 6+. The uranyl ion forms compounds that are soluble in water, so U is a mobile element under oxidizing conditions. In contrast to U, Pb (Z = 82) is in period 6 and is a group 14 post-transitional metal. It is insoluble in water, but is a chalcophile element because it reacts with sulfur. It forms Pb2+ and Pb4+ ions with ionic radii of 1.32 Å and 0.91 Å respectively, so Pb ions cannot substitute for U ions in minerals.
In the course of the earth’s history, during partial melting of the rocks in the earth’s mantle U was concentrated in the liquid (melt) phase and thus became incorporated into the more silica-rich products. Therefore, the progressive geochemical differentiation of the earth’s upper mantle has enriched the rocks of the earth’s continental crust in U compared to those of the upper mantle. At an average of 1.3 ppm U is the 51st most abundant element in the earth’s crust, whereas Pb is regarded as quite a common element in the earth’s crust with an average of 11 ppm (Rudnick and Gao 2005). The concentrations of U and Pb increase from basaltic rocks (0.5 ppm U and 4 ppm Pb) to granites (5 ppm U and 23 ppm Pb) (Faure and Mensing 2005, 215). The concentrations of U in the common rock-forming silicate minerals are uniformly low, on the order of a few ppm or less. Instead, U occurs primarily in certain accessory minerals in which it is either a major constituent or replaces other elements. These minerals include uraninite, zircon, baddeleyite, monazite, apatite, and sphene (titanite).
All six naturally occurring U isotopes are unstable and decay. Of these, 238U is the dominantly abundant isotope in natural U. It and 235U, the next most abundant isotope, are the starting radioisotopes in two decay chains or series (figs. 1 and 2), with 234U one of the early steps in the 238U decay chain. There are also several other trace U isotopes. 239U is formed when 238U undergoes spontaneous fission, releasing neutrons that are captured by other 238U atoms. 237U is formed when 238U captures a neutron but emits two more, which then decays to 237Np (neptunium). And then 233U is formed in the decay chain of that 237Np. 233U is also made from 232Th by neutron bombardment, usually in a nuclear reactor.
On the other hand, Pb has four stable isotopes, three of which (206Pb, 207Pb, and 208Pb) are the end members of decay chains (238U, 235U, and 232Th respectively). Only stable 204Pb has no radioactive precursor from which it is derived, and thus it is often called common Pb. Thus, the isotopic concentration of Pb in a natural rock sample depends on how much U and Th are also present. For example, the relative amount of 208Pb can range from 52.4% in normal samples to 90% in thorium ores. Similarly, the ratios of 206Pb and 207Pb to 204Pb increase in different samples, since the former two are supplemented by radioactive decay of U and the latter is not. For this reason, the atomic weight of lead is given to only one decimal place. Both 214Pb and 210Pb are short-lived intermediates in the 238U decay chain (fig. 1), while 211Pb and 212Pb are short-lived intermediates in the 235U and 232Th decay chains respectively (fig. 2). Lastly, very minute traces of 209Pb are also present from the cluster decay of 223Ra, one of the daughter products of natural 235U (fig. 2). Hence, natural Pb consists of not only the four stable isotopes, but also minute traces of another five short-lived radioisotopes.
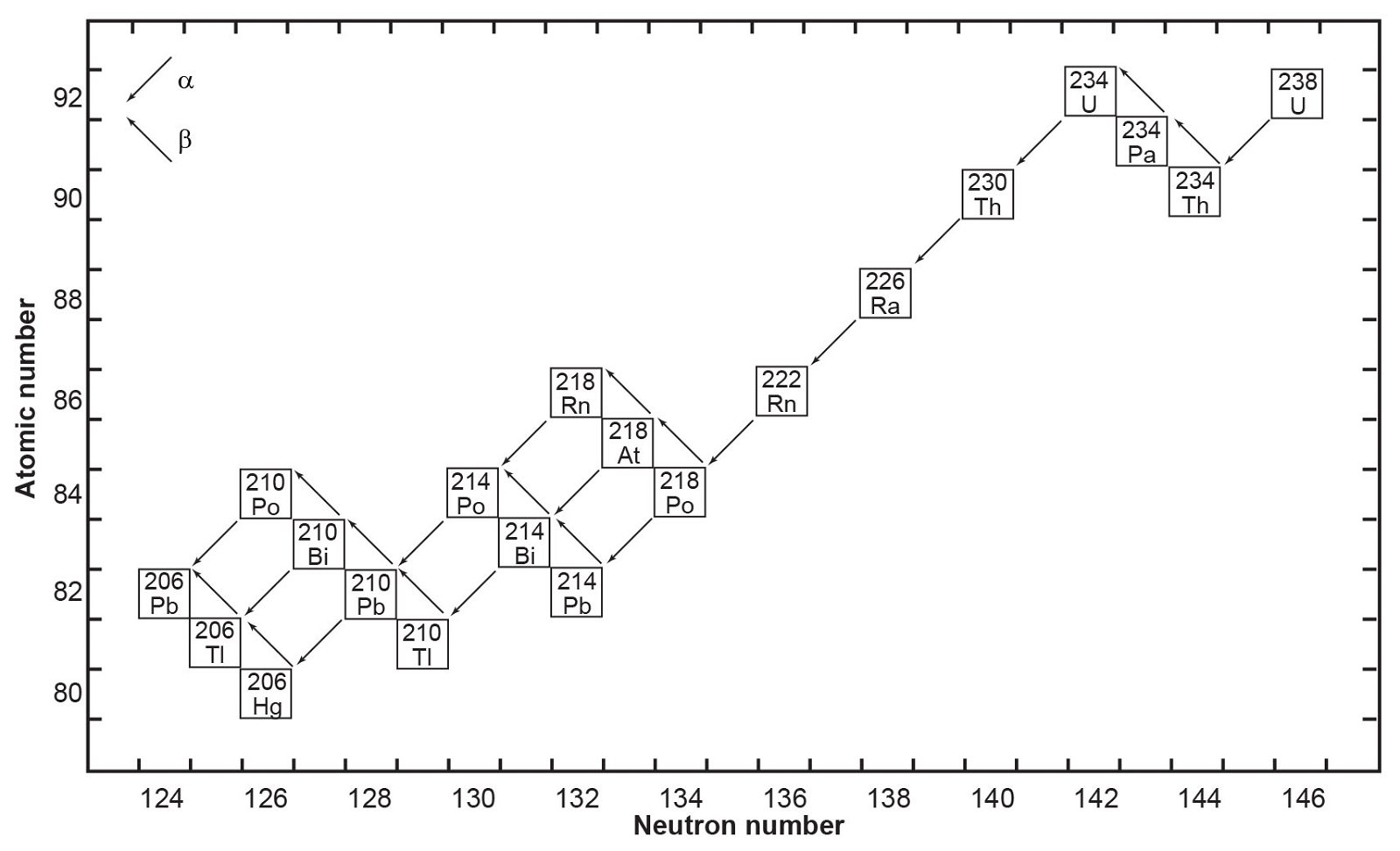
Fig.1. The decay chain of 238U resulting from the successive emission of α-particles and β-particles from intermediate isotopes as indicated (after Faure and Mensing 2005). The final decay product is stable 206Pb.
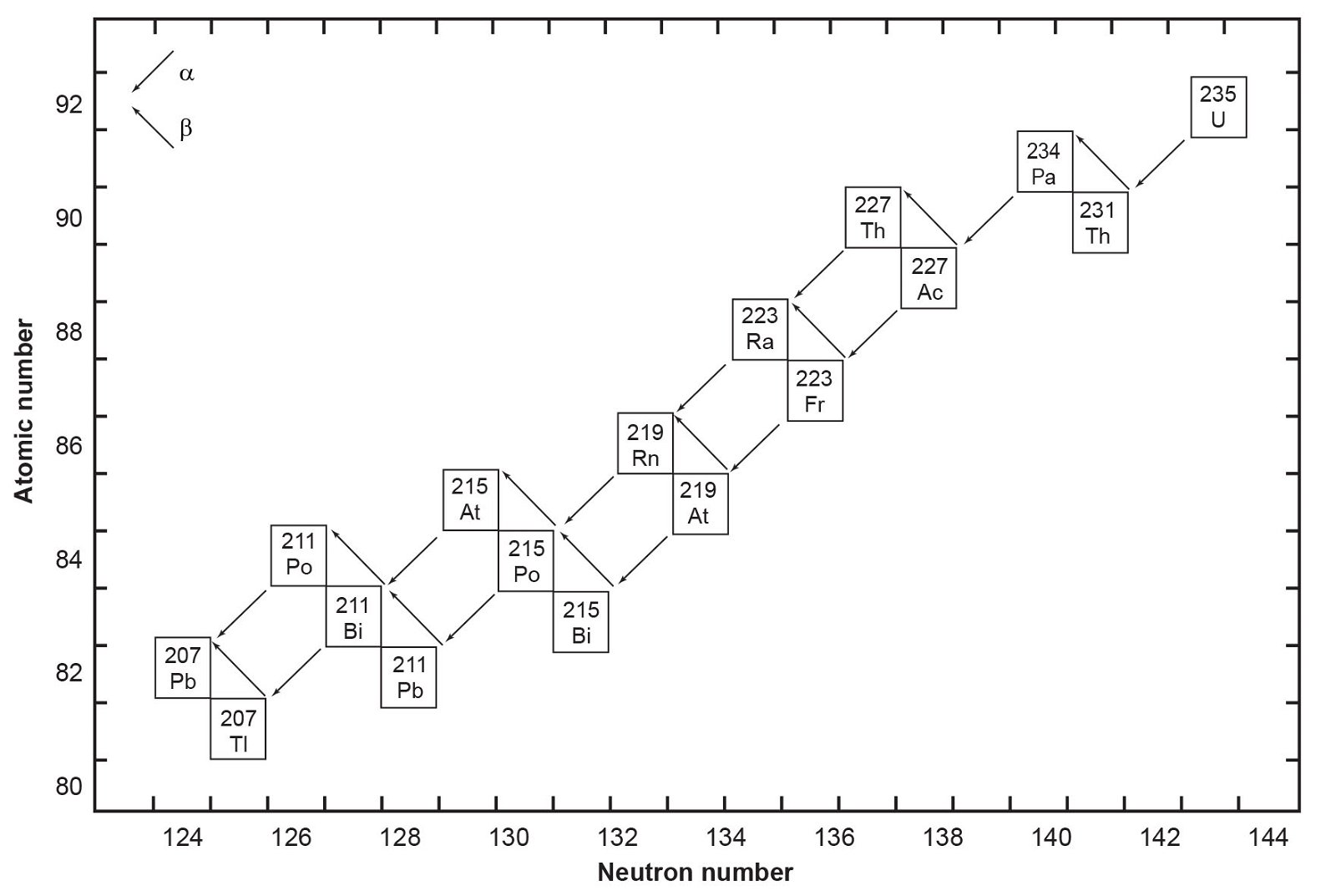
Fig. 2. The decay chain of 235U resulting from the successive emission of α-particles and β-particles from intermediate isotopes as indicated (after Faure and Mensing 2005). The final decay product is stable 207Pb.
Primordial Pb, which comprises the amounts of the isotopes 204Pb, 206Pb, 207Pb, and 208Pb at the time the earth formed, has been defined as the Pb isotopic composition of troilite (FeS) in the Canyon Diablo iron meteorite (Chen and Wasserburg 1983; Tatsumoto, Knight, and Allègre 1973). It is postulated to have been mostly “created” as a result of repetitive rapid and slow neutron capture processes occurring in stars. Yet there are serious questions about the so-called r-process in supernova which is postulated to generate all the elements heavier than Fe (Thielemann et al. 2011). Thus, it should be noted that this is not an absolute value, but merely an artifact of the reigning popular model for the naturalistic formation of the universe and its component stars and planetary systems.
238U and 235U Decay
The decay of the uranium isotopes 238U and 235U to the stable lead isotopes 206Pb and 207Pb respectively is the basis for the several most important methods of radioisotope dating. These not only derive from the transformation of 238U and 235U to 206Pb and 207Pb respectively, but also derive from the time-dependent “evolution” of common lead 204Pb from the decay of the intermediate daughters of 238U and 235U, and from the resulting isotopic composition of the accumulating daughter He (helium). Of course, 204Pb is not produced from 238U or 235U decay. However, 204Pb is assumed to be primordial and thus is hypothetically used as an indicator of the 206Pb, 207Pb, and 208Pb present due to radioactive decay. Age determinations of rocks based on the decay of U and resulting accumulation of Pb and He were first attempted in the early years of the twentieth century by Rutherford (1906) and Boltwood (1907). Subsequently, Holmes (1913) used chemical U-Pb and U-He dates to propose the first geological timescale based on radioisotope dating in his book on the age of the earth.
The invention of the first mass spectrometer by Thomson (1911) was followed by the work of Dempster (1918) and Ashton (1919), who designed the mass spectrographs which they used in subsequent years to discover the naturally occurring isotopes of most of the elements in the periodic table and to measure their masses and abundances. The design of mass spectrographs was further improved in the 1930s, but it was the mass spectrometers based on a design by Nier (1940) that made possible the measurement and interpretation of variations in the isotopic composition of certain elements in natural materials such as minerals and rocks. Modern mass spectrometers follow his design and achieve a high level of accuracy and reliability of operation which enable isotope ratios to be measured for radioisotope dating, such as that based on the isotopic composition of Pb due to the decay of U to Pb, but also on the isotope ratios of common Pb. As a result of continuing refinement of the analytical procedures and of the sophistication of the instrumentation, the U-Pb and Pb-Pb methods of radioisotope dating are now regarded as the most precise and accurate geochronometers for determining the ages of terrestrial and extra-terrestrial minerals and rocks. As already indicated, U has three naturally occurring isotopes, 238U, 235U, and 234U, all of which are radioactive. The decay of 238U gives rise to what is called the uranium series, which includes 234U as one of the intermediate daughters and ends in stable 206Pb (fig. 1). The decay of 238U to 206Pb can be summarized by the equation
where Q = 47.4 MeV per atom or 0.71 calories per gram per year (Wetherill 1966). Each atom of 238U that decays produces one atom of 206Pb by emission of eight α-particles and six β-particles. The parameter Q represents the sum of the decay energies of the entire series in units of millions of electron volts and calories of heat produced per gram per year. Several intermediate daughters in this series (fig. 1) undergo branched decay involving the emission of either an α-particle or a β-particle. The chain therefore splits into separate branches but 206Pb is the stable end-product of all possible decay paths.
The decay of 235U gives rise to what is called the actinium series (fig. 2), which ends with stable 207Pb after emission of seven α-particles and four β-particles, as summarized by the equation
where Q = 45.2 MeV per atom or 4.3 calories per gram per year (Wetherill 1966). This series also branches as shown in Fig. 2.
In spite of there being 33 isotopes of 12 elements formed as intermediate daughters in these two decay series (not counting 4He), none is a member of more than one series. In other words, each decay chain always leads through its unique set of intermediate isotopes to the formation of a specific stable Pb isotope. The decay of 238U always produces 206Pb, and 235U always produces 207Pb.
The half-lives of 238U and 235U are very much longer than those of their respective intermediate daughter isotopes. Therefore, these decay series satisfy the prerequisite condition for the establishment of secular equilibrium, provided none of the intermediate daughters escaped from the U-bearing mineral or were added from external sources (Faure and Mensing 2005, 218). When secular equilibrium exists in a U-bearing mineral because it is a closed system, the decay rates of the intermediate daughters are equal to those of their respective parents, and thus the production rate of the stable daughter at the end of the decay chain is equal to the decay rate of its parent at the head of that chain. Therefore, the decay of 238U and 235U in minerals in which secular equilibrium has established itself can be directly linked qualitatively to the respective 206Pb and 207Pb isotopes. As a result, the growth of these radiogenic Pb isotopes can be described by means of equations (1) and (2), which are similar to the equations used to represent the decay of 87Rb to 87Sr and 147Sm to 143Nd.
The U-Pb Dating Methods
The accumulation of stable daughter atoms from the decay of parent atoms over time is expressed by the equation known as the law of radioactivity, namely
where D* is the number of measured stable radiogenic daughter atoms, N is the number of measured parent atoms remaining, λ is the decay constant (decay rate), and t is the time since decay of the parent atoms began (Faure and Mensing 2005). Since D* and N can be measured in a mineral, then if λ is known the equation can be solved for t, which is thus declared to be the age of the mineral. Thus, the accumulation of stable radiogenic 206Pb and 207Pb by decay of their respective parents 238U and 235U in a mineral is governed by equations derivable from equation (3) as follows
where λ1 and λ2 are the decay constants of 238U and 235U respectively; 238U/204Pb and 235U/204Pb are ratios of these isotopes calculated from the measured concentrations of U and Pb in the mineral; and the subscript i refers to the unknown initial values of the 206Pb/204Pb and 207Pb/204Pb ratios.
To date U-bearing minerals by the U-Pb methods, the concentrations of U and Pb are measured by an appropriate analytical technique (usually isotope dilution), and the isotopic composition of Pb is determined by using a solid-source mass spectrometer, an ion-probe mass spectrometer, or an ICP mass spectrometer. The U-Pb dates are calculated by means of equations (4) and (5) being solved for t using assumed values of the initial isotope ratios of Pb (for example, Ludwig 1993) as follows
These are known as 206Pb and 207Pb model ages respectively. They are independent of each other, but will be concordant (that is, agree with each other) if the mineral samples satisfy the conditions for dating (Faure and Mensing 2005, 218–219):
- the mineral has remained closed to U and Pb, and all the intermediate daughters throughout its history;
- correct values are used for the initial Pb isotope ratios;
- the decay constants of 238U and 235U are known accurately;
- the isotopic composition of U is normal and has not been modified by isotope fractionation or by occurrence of a natural chain reaction based on induced fission of 235U; and
- all analytical results are accurate and free of systematic errors.
The assumption that the samples being dated remained closed to U, Pb, and all intermediate daughters throughout their history “is satisfied only in rare cases because U is a mobile element in oxidizing environments and therefore tends to be lost during chemical weathering” (Faure and Mensing 2005, 219, emphasis in the original). In addition, the emission of α-particles causes radiation damage to the crystal structures of the U-hosting minerals, which facilitates the loss of Pb and the other intermediate daughters in both decay chains. Consequently, U-Pb dates for rocks and minerals are rarely concordant, so procedures have been devised to overcome that problem.
The choice of the initial Pb isotope ratios would seem to only be a problem for dating rocks and minerals that have low U/Pb ratios and additionally are young. It is claimed that the numerical values of the initial Pb isotope ratios do not appear to significantly affect the calculated U-Pb ages of Precambrian rocks and minerals having high U/Pb ratios because their present Pb isotope ratios in most cases reach large values.
The decay constants and half-lives of 238U and 235U were fixed by the International Union of Geological Sciences (IUGS) Subcommission of Geochronology in 1975 (Steiger and Jäger 1977). At the same time a value of 137.88 was adopted for the 238U/235U ratio. Since then these values have been used in almost all U-Pb age calculations so as to avoid any potential confusion by the use of different values. It has been continually claimed that the numerical values of the 238U and 235U decay constants and half-lives are probably more accurately known that those of other long-lived radionuclides because of their importance in the nuclear industry. Therefore, refractory U-bearing minerals such as zircon (ZrSiO4) that often yield concordant U-Pb ages have been used to refine (that is, adjust) the decay constants of other radionuclides used in geochronology (Begemann et al. 2001; Snelling 2014a, b, 2015a, b, 2016).
It should be mentioned here that decay rates are not just measured and expressed by the parameter known as the decay constant (λ), but also by the parameter called the half-life (t½). The decay constant can be defined as the probability per unit time of a particular nucleus decaying, whereas the half-life is the time it takes for half of a given number of the parent radionuclide atoms to decay. The two quantities can be almost used interchangeably, because they are related by the equation:
The issue of the abundances of the U isotopes and thus the adopted value of the 238U/235U ratio has already been discussed in great detail by Snelling (2017), so further comment is not warranted here. Suffice it to say, real differences in the isotopic composition of terrestrial and extra-terrestrial U have been reported in the past decade. So until very recently there has been no compelling evidence not to base age determinations of terrestrial and lunar rocks and minerals, and of meteorites and their minerals, by the U-Pb method on a value of 137.88 for the present-day 238U/235U ratio.
It is claimed that the effect of Pb loss on U-Pb dates can be minimized by calculating a date based on the 207Pb/206Pb ratio which is supposed to be insensitive to recent Pb loss provided that the Pb which was lost from the mineral had the same isotopic diffusion rate as the Pb which remained, that is, there has been no isotopic fractionation. The relationship between the 207Pb/206Pb ratio and time results from the difference in the half-lives of 238U and 235U. The desired equation is obtained by combining equations (4) and (5) above:
This equation has several interesting properties (Faure and Mensing 2005, 219–220):
it involves the 235U/238U ratio which at 1/137.88 has been regarded as a constant for all U of normal isotopic composition on and in the earth, the moon, Mars, and meteorites at the present time.
the equation does not require knowledge of the concentrations of U and Pb and involves only isotope ratios of Pb.
-
the left hand side of equation (9) is equal to the 207Pb/206Pb ratio of radiogenic Pb:
(10)where the asterisk * identifies the radiogenic isotopes.
equation (9) cannot be solved for t by algebraic means because it is transcendental, but it can be solved by iteration and by interpretation in a table.
A difficulty arises in the solution of equation (9) when t = 0, because it yields the indeterminate result 0/0. It is claimed that this difficulty is overcome by means of l’Hôpital’s rule (Faure and Mensing 2005, 220), which requires that the differentiated functions in the ratio are differentiable over the entire open interval in question, that is, over millions to billions of years. However, it is questionable whether this is a proper application of l’Hôpital’s rule. This is because the decay rates of 235U and 238U are not equal, and therefore they are each functions of time and thus the ratio must be a function of time. Hence the right side of equation (9) is not in a form amenable to l’Hôpital’s rule, that is, there are four functions of time involved in the open interval 0 < t < ta. Nevertheless, applying this rule, the value of (207Pb/206Pb)* at the present time (t = 0) is
Equation (11) indicates that the (207Pb/206Pb)* which forms by the decay of 238U and 235U over the time interval equalling the age of the mineral is equal to the rates of decay of these two U isotopes at the present time. Substituting into equation (11) the relevant values for the 235U/238U ratio, and the decay constants λ1 and λ2, yields a value at the present time (t = 0) for (207Pb/206Pb)* of 0.04604. (Parenthetically, this procedure thus predicts a 207Pb/206Pb ratio of 0.04604 at the time of creation of the earth.) However, of time. Thus it can be argued that ratio should be 0/0 at t = 0, not 0.0464, since that is supposedly the beginning of the earth’s formation. Furthermore, the right side of equation (11) is evaluated in the present, while the left side is evaluated at t = 0.
The numerical values of (eλ1t − 1) and (eλ2t − 1) are listed in Table 1 and yield the (207Pb/206Pb)* ratios for increasing values of t ranging from t = 0 to t = 4.6 Byr. This table can be used to solve equation (9) for t by linear interpolation based on the (207Pb/206Pb)* ratio calculated from equation (10). Conversely, by determining the (207Pb/206Pb)* ratio in a mineral from measurements of its Pb isotope ratios, the age (t) of the mineral can be calculated by linear interpolation between the (207Pb/206Pb)* ratio values in Table 1. This is known as the 207Pb- 206Pb model age. However, since the equation is of the form x(t) = y(t) and the left side of the equation is differentiated by the independent variable, it is questionable whether it is logical to then assume that x(t) = y(t) dt is still valid.
| t, × 109 y | eλ1t − 1 | eλ2t − 1 | (207Pb/206Pb)* |
|---|---|---|---|
| 0 | 0.0000 | 0.0000 | 0.04604 |
| 0.2 | 0.0315 | 0.2177 | 0.05012 |
| 0.4 | 0.0640 | 0.4828 | 0.05471 |
| 0.6 | 0.0975 | 0.8056 | 0.05992 |
| 0.8 | 0.1321 | 1.1987 | 0.06581 |
| 1.0 | 0.1678 | 1.6774 | 0.07250 |
| 1.2 | 0.2046 | 2.2603 | 0.08012 |
| 1.4 | 0.2426 | 2.9701 | 0.08879 |
| 1.6 | 0.2817 | 3.8344 | 0.09872 |
| 1.8 | 0.3221 | 4.8869 | 0.11004 |
| 2.0 | 0.3638 | 6.1685 | 0.12298 |
| 2.2 | 0.4067 | 7.7292 | 0.13783 |
| 2.4 | 0.4511 | 9.6296 | 0.15482 |
| 2.6 | 0.4968 | 11.9437 | 0.17436 |
| 2.8 | 0.5440 | 14.7617 | 0.19680 |
| 3.0 | 0.5926 | 18.1931 | 0.22266 |
| 3.2 | 0.6428 | 22.3716 | 0.25241 |
| 3.4 | 0.6946 | 27.4597 | 0.28672 |
| 3.6 | 0.7480 | 33.6556 | 0.32634 |
| 3.8 | 0.8030 | 41.2004 | 0.37212 |
| 4.0 | 0.8599 | 50.3878 | 0.42498 |
| 4.2 | 0.9185 | 61.5752 | 0.48623 |
| 4.4 | 0.9789 | 75.1984 | 0.55714 |
| 4.6 | 1.0413 | 91.7873 | 0.63930 |
Although U occurs in a large number of minerals, only a few are suitable for dating by the U-Pb methods. To be useful for dating, a mineral must be retentive with respect to U, Pb and the intermediate daughters, and it should be widely distributed in a variety of rocks. The minerals that satisfy these conditions include zircon, baddeleyite, monazite, apatite, and sphene (titanite). All of these minerals contain trace amounts of U but low concentrations of Pb, giving them high U/Pb ratios favourable for dating. For example, concentrations of U in zircons range from a few hundred to a few thousand parts per million and average 1350 ppm (Faure and Mensing 2005, 221). The presence of U in zircon is due to the isomorphous substitution within the zircon crystal lattice of U4+ (ionic radius 1.05 Å) for Zr4+ (0.87 Å), although this substitution is limited by the differences in their ionic radii and may well be an exothermic reaction due to the substitution sites having to expand by 20%. However, whereas U4+ is admitted into zircon crystals, Pb2+ is regarded as being excluded because of its large ionic radius (1.32 Å) and its low charge (2+). Therefore, zircons are supposed to contain very little initial Pb at their time of formation and have high U/Pb ratios. This appears to enhance their sensitivity as a geochronometer, so zircons have for several decades become increasingly used for dating via the U-Pb methods.
The Wetherill Concordia
The effect of the loss of Pb or U and the gain of U on U-Pb dates of minerals can be compensated by a graphical procedure developed by Ahrens (1955) and Wetherill (1956, 1963). Equations (4) and (5), which govern the time-dependent increase of the 206Pb/204Pb and 207Pb/204Pb ratios of U-bearing minerals or rocks, can be rearranged to yield ratios of radiogenic 206Pb to 238U and of radiogenic 207Pb to 235U:
where the asterisk * is used to identify the radiogenic origin of the Pb isotopes. These equations assume that there is no 206Pb or 207Pb present when t = 0. Yet this begs the question as to whether t = 0 at the formation of the earth and solar system, or when the mineral forms and remains a closed system.
The values of eλ1t − 1 and eλ2t − 1 for different values of t are listed in Table 1 and were used to plot the curve in Fig. 3. The coordinates of all points on this curve are the 206Pb*/238U and 207Pb*/235U ratios that yield concordant U-Pb dates. Therefore, the curve in Fig. 3 is known as the concordia and is associated with its inventor (Wetherill 1956, 1963) in order to distinguish it from a different concordia diagram developed later by others. U-bearing minerals that contain no radiogenic 206Pb* and 207Pb* yield t = 0, while those containing radiogenic 206Pb* and 207Pb* will yield U-Pb ages of 1.0 Byr, 1.5 Byr and so on, located sequentially along the concordia curve.
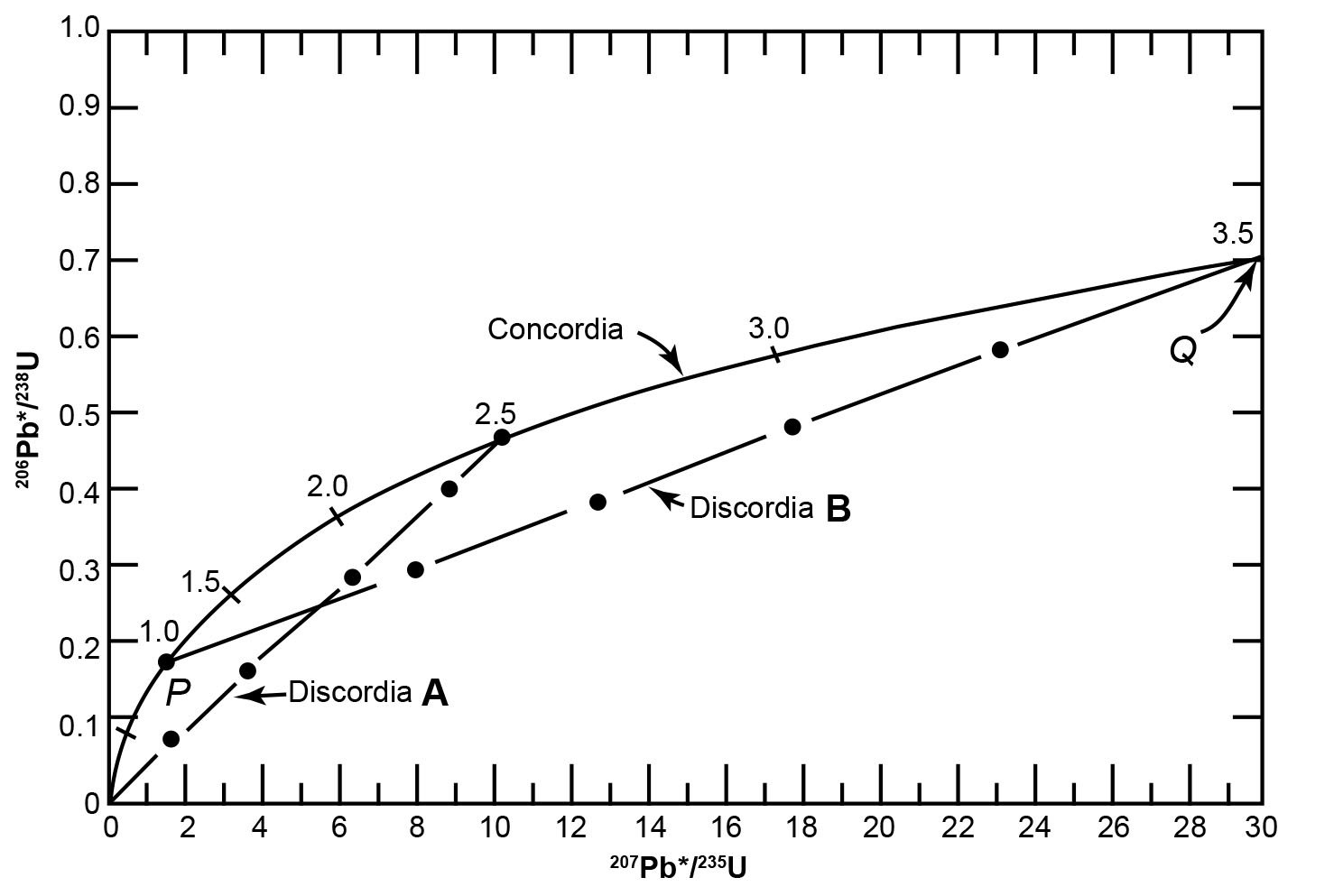
Fig. 3. The concordia diagram used for the interpretation of U-bearing minerals that lost radiogenic Pb and therefore yield discordant dates, as developed by Wetherill (1956, 1963) (after Faure and Mensing 2005).
Fig. 3 shows a hypothetical history of zircon grains that originally crystallized from a magma. At the time of crystallization, the zircons contained no radiogenic Pb and so plotted at the origin of the concordia diagram. During the subsequent 2.5 Byr the 206Pb*/238U and 207Pb*/235U ratios of the zircons increased by decay of 238U and 235U and the simultaneous increase of both ratios causes them to move upwards along the concordia. After 2.5 Byr there was an episode of thermal metamorphism during which some of the zircon grains lost all the radiogenic Pb they had accumulated (due to the heat causing the mobility of the Pb atoms), and they therefore now plot back at the origin (t = 0). Yet it could be equally argued that these zircon grains may have lost more U than radiogenic Pb because U is more mobile. Furthermore, this happening introduces a discontinuity in the equations describing the process and hence could invalidate the application of l’Hôpital’s rule to the original equation. Meanwhile, the other grains lost varying amounts of radiogenic Pb so they plot on a straight-line chord, labelled as discordia A on Fig. 3 because all the zircon grains on this chord would yield discordant U-Pb dates. At the end of this short episode of thermal metamorphism the U in all the zircon grains did not stop nuclear decay and so the grains resumed accumulating radiogenic Pb. At the present time 1 Byr after the episode of thermal metamorphism, the zircon grains that had previously lost all their radiogenic Pb have moved 1 Byr up along the concordia, while the other grains that had previously lost varying amounts of radiogenic Pb have maintained their linear relationship to one another. The net result is that the zircon grains now plot along discordia B in Fig. 3, extending from 1 Byr (the time elapsed since the thermal metamorphism) to 3.5 Byr (2.5 Byr + 1 Byr). Thus, at 1 Byr after the episode of thermal metamorphism (which occurred at 2.5 Byr after the crystals formed) the zircon grains that previously defined discordia A now form discordia B, which intersects the concordia at two points, labelled P and Q in Fig. 3. The coordinates of point Q represent concordant U-Pb dates of 3.5 Byr which represents the time elapsed since the original crystallization of the zircon grains that now define discordia B.
Furthermore, the coordinates of point P yield concordant U-Pb dates of 1 Byr, but the interpretation of that date depends on the circumstances. If the loss of radiogenic Pb did occur during the short episode of thermal metamorphism, then the date of 1 Byr at point P is the time elapsed since that episode. This is called episodic loss of radiogenic Pb from the zircon grains. At the same time the thermal metamorphism should have caused loss of radiogenic 40Ar from other minerals in the same rock, which should thus yield a K-Ar date also of 1 Byr. Alternatively, radiogenic Pb loss may have occurred by continuous diffusion at elevated temperature. In that case, the trajectory of the U-Pb system in the zircons would follow a straight line that became non-linear near the origin (t = 0). As a result, linear extrapolation of discordias would yield a lower intercept with concordia that corresponds to a fictitious date. Therefore, the date calculated for the lower intercept point P of discordia B in Fig. 3 must be confirmed by a K-Ar date for another mineral in the same rock before it can be interpreted as the age of an episode of thermal metamorphism.
Thus the concordia diagram can indicate the U-bearing minerals that plot on a discordia line were altered. As well as loss of radiogenic Pb from a mineral, a discordia may represent a gain or loss of parent U. However, on this concordia diagram the gain of Pb by the mineral is not predictable unless the isotopic composition of the new Pb can be specified. The concordia model also includes a further constraint that the Pb loss must occur without discrimination between the Pb isotopes on the basis of their masses (that is, fractionation). Thus, it has been shown by Faure and Mensing (2005) how both Pb loss and U gain will cause a mineral’s grains to plot along a discordia below the date of their original formation and yield younger discordant U-Pb dates. On the other hand, a loss of U from the mineral will cause its grains to plot along that same discordia above the date of their original formation and yield older discordant U-Pb dates.
The Tera-Wasserburg Concordia
The U-Pb dates of some lunar rocks were found to be significantly older than the Rb-Sr and K-Ar dates yielded by the same rocks (Tatsumoto and Rosholt 1970, compared with Tera and Wasserburg 1972, 1973). For example, a lunar basalt yielded 238U-206Pb and 235U-207Pb model ages of 4.24 Byr and 4.27 Byr respectively compared to Rb-Sr and K-Ar dates of only 3.88 Byr (Tera and Wasserburg 1972). The postulated reason for this discrepancy is that these lunar rocks contain excess radiogenic 206Pb and 207Pb that was incorporated into these lunar basalts at the time of crystallization, but no explanation is given as to where this excess radiogenic Pb came from. Tera and Wasserburg (1972) therefore devised a new concordia that does not require prior knowledge of the initial 206Pb/204Pb and 207Pb/204Pb ratios.
The number of 206Pb and 207Pb atoms in a unit weight of U-bearing rocks or minerals can be expressed by the equations:
where 206Pbi and 207Pbi are the initial 206Pb and 207Pb respectively. Tera and Wasserburg (1972) used these equations to define a concordia in parametric form where the x-coordinate is derived from equation (14) as follows:
and the y-coordinate is obtained by combining equations (14) and (15) as follows:
The concordia is constructed by solving equation (16) (x-coordinate) and equation (17) (y-coordinate) for selected values of t. However, in order to plot two such parameters against each other, a postulated relation must exist between them. Thus it could be questioned as to whether these two parameters are actually in a linear relationship to begin with. Nevertheless, the results are listed in Table 2. The resulting graph in Fig. 4 is the locus of all points representing U-Pb systems that yield concordant dates.
| t, Byr | x | y |
|---|---|---|
| 0.2 | 31.746 | 0.05012 |
| 0.4 | 15.625 | 0.05575 |
| 0.6 | 10.256 | 0.05992 |
| 0.8 | 7.570 | 0.06581 |
| 1.0 | 5.959 | 0.0725 |
| 1.2 | 4.887 | 0.0801 |
| 1.4 | 4.122 | 0.0887 |
| 1.6 | 3.549 | 0.0987 |
| 1.8 | 3.104 | 0.1100 |
| 2.0 | 2.748 | 0.1229 |
| 2.2 | 2.458 | 0.1378 |
| 2.4 | 2.216 | 0.1548 |
| 2.6 | 2.012 | 0.1743 |
| 2.8 | 1.838 | 0.1968 |
| 3.0 | 1.687 | 0.2226 |
| 3.2 | 1.555 | 0.2524 |
| 3.4 | 1.439 | 0.2867 |
| 3.6 | 1.336 | 0.3263 |
| 3.8 | 1.245 | 0.3721 |
| 4.0 | 1.162 | 0.4249 |
| 4.2 | 1.088 | 0.4862 |
| 4.4 | 1.021 | 0.5571 |
| 4.6 | 0.9603 | 0.6393 |
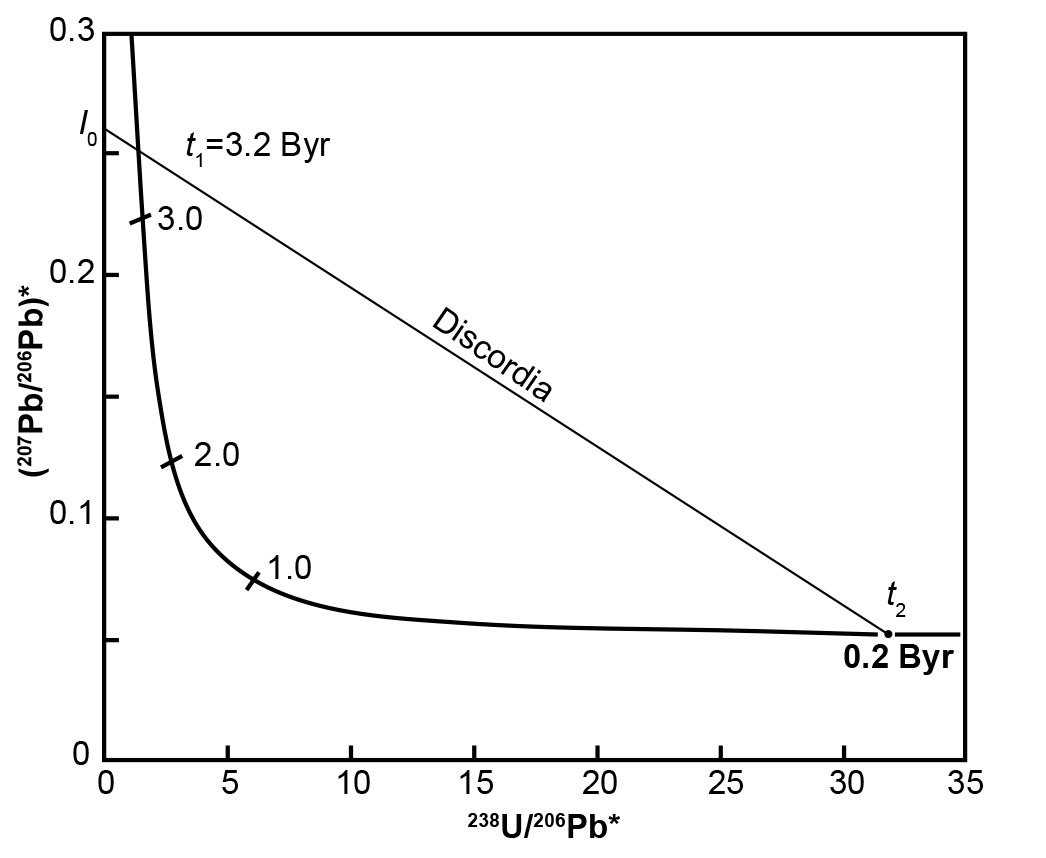
Fig. 4. The Tera-Wasserburg concordia based on equation (16) (x-coordinate) and equation 17) (y-coordinate) plotted from values in Table 2, assuming that λ1(238U) = 1.55125 × 10-10yr-1, λ2(235U) = 9.8485 × 10-10yr-1, and 238U/235U = 137.88 (after Faure and Mensing 2005).
The discordia line in Fig. 4 intersects the Tera-Wasserburg concordia at two points corresponding to dates t1 (3.2 Byr) and t2 (0.2 Byr). Extrapolation of this discordia line beyond t1 yields an intersection point I0 on the y-axis where 238U/206Pb* = 0. Obviously, this means that the 206Pb* concentration must be non-zero when there is no 238U, and calculating the relevant values infers that the 206Pb* is approximately four times larger than the 207Pb* concentration. In any case, the numerical value of I0 is the radiogenic 207Pb/206Pb ratio that formed in the interval of time between t1 and t2 (Tera and Wasserburg 1974) as follows:
where λ1 and λ2 are the decay constants of 238U and 235U respectively, and t1 and t2 are upper and lower intersections with the discordia as depicted in Fig. 4.
The U-Pb system whose postulated geological history is depicted in Fig. 4 originally contained no radiogenic Pb, that is, 238U/206Pb* = ∞ when it formed at t1 = 3.2 Byr. Subsequently, radiogenic 207Pb and 206Pb accumulated by decay of 235U and 238U respectively until the system apparently recrystallized or differentiated at t2 = 0.2 Byr. The radiogenic 207Pb/206Pb ratio of the Pb that had accumulated from t1 = 3.2 Byr to t2 = 0.2 Byr is equal to I0 in Fig. 4 and is expressed by equation (18). If a U-free mineral (for example, plagioclase) formed during the recrystallization event at t2, it would contain Pb whose radiogenic 207Pb/206Pb ratio is equal to I0.
The postulated U-bearing system represented by t2 on the Tera-Wasserburg concordia in Fig. 4 was apparently Pb-free at the end of the metamorphic event (that is, 238U/206Pb* = ∞). All the Pb it contains at the present time apparently formed by decay of the U isotopes after the end of the recrystallization event at t2 = 0.2 Byr. This interpretation implies that both t1 and t2 are valid dates in the geological history of a volume of U-bearing rocks. Keep in mind that t1 and t2 are obtained as result of where the discordia plotted on the Tera-Wasserburg diagram from the U and Pb isotope analyses of the rock unit being investigated intersects the Tera-Wasserburg concordia, as depicted in Fig. 4.
Alternately, the radiogenic 207Pb/206Pb ratio represented by I0 may be the result of a complex process unrelated to the U-Pb system that recrystallized at t2. In that case, the discordia is the locus of U-Pb systems that formed by mixing of two components. One of the components is I0 and the other component is the U-Pb system represented by the point of intersection at t2 in Fig. 4. In that case, the date derived from the coordinates of point t1 has no geological significance (Tera and Wasserburg 1974).
A follow-on example illustrates how the Tera-Wasserburg concordia diagram has been utilized to obtain a corrected age for a rock with discordant U-Pb model ages. One of the rock samples obtained by the Apollo 14 mission from the Fra Mauro region of the Moon was lunar basalt 14053. This sample yielded highly discordant and improbable whole-rock U-Pb model ages of 5.60 Byr (238U-206Pb), 5.18 Byr (235U-207Pb), and 5.01 Byr (207Pb/206Pb), corrected for the postulated presence of primeval Pb which the moon supposedly inherited from the solar nebula (Tera and Wasserburg 1972). The same sample had yielded an internal (mineral and whole-rock) Rb-Sr isochron date of 3.88 ± 0.04 Byr (Papanastassiou and Wasserburg 1971). Furthermore, the same sample had been dated by Turner et al. (1971) by the 40Ar*/39Ar method applied to both the whole-rock and plagioclase. The partial-release spectra indicated well-defined plateau dates of 3.95 Byr (whole rock) and 3.93 Byr (plagioclase), using then current recommended decay constants.
The U-Pb data reported by Tera and Wasserburg (1972) for lunar basalt 14053 define a discordia line that intersects the y-axis (238U/206Pb* = 0) at (207Pb/206Pb)* = 1.46, as depicted in Fig. 5. The slope of the discordia line is −0.88366 based on an unweighted linear regression of three data points representing two whole-rock and one magnetite (the magnetic fraction) analyses. The slope m of the discordia is related to the initial (207Pb/206Pb)* ratio and to the age of the U-Pb system by the equation:
This equation was solved graphically by Tera and Wasserburg (1972) for (207Pb/206Pb)i = 1.46 for values of t between 3.87 and 4.00 Byr. Their graph indicates that a slope of −0.88366 corresponds to a date of 3.91 Byr, which represents the intersection point of the discordia with the Tera-Wasserburg concordia in Fig. 5. A more accurate date could be obtained by interpolating in a table values of the slope for selected values of t or by numerical iteration.
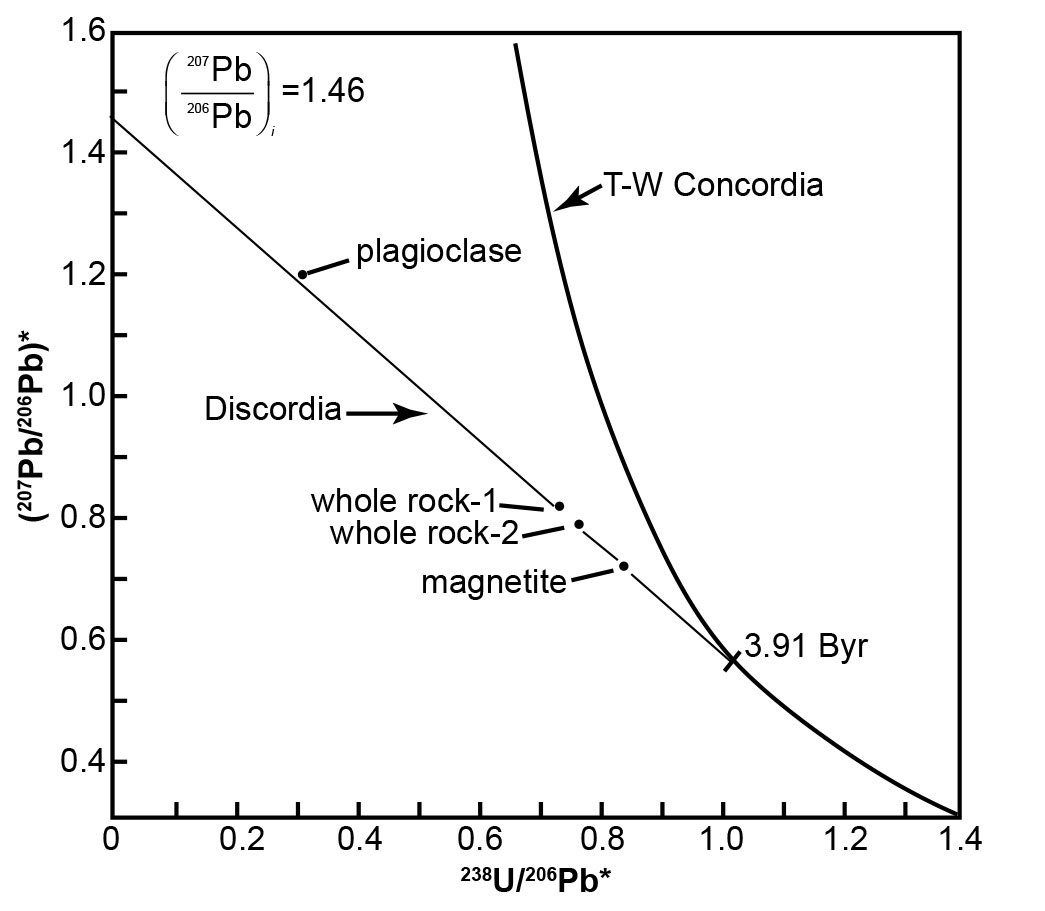
Fig. 5. Tera-Wasserburg concordia diagram for U-Pb data of lunar basalt 14053 (after Tera and Wasserburg 1972). The slope of the discordia line is m = −0.88366 and the intercept on the y-axis for 238U/206Pb = 0 is 1.46. The date that corresponds to the intersection point of the discordia with the Tera-Wasserburg concordia was determined graphically from equation (19).
This interpretation of the U-Pb data for lunar basalt 14053 by means of the Tera-Wasserburg concordia yielded a date that is in good agreement with the Rb-Sr and 40Ar*/39Ar dates for this same rock. The essential feature of this concordia is that it permits an explicit determination of the radiogenic 207Pb/206Pb ratio at 238U/206Pb* = 0 without requiring an estimate of the initial isotope ratios of Pb at the time of crystallization of the basalt.
Pb-Pb Isochron Dating
Equations (4) and (5) above describe the accumulation of the radiogenic 206Pb and 207Pb from 238U and 235U respectively. The same equations can be used with multiple samples to plot independent isochrons. The slopes of the 238U-206Pb and 235U-207Pb isochrons yield dates that are concordant only when the samples remained closed to Pb diffusion and had identical initial Pb isotopic ratios. However, in most cases, U-Pb isochrons based on whole-rock samples have not been successful, primarily because rocks are exposed to chemical weathering and lose a significant fraction of U. Thus, the U-Pb isochron method of dating igneous and metamorphic rocks composed of silicate minerals does not work in most cases because of the variable losses of U by chemical weathering, which occurs not only at the earth’s surface, but also in the subsurface where rocks are in contact with oxygenated groundwater.
On the other hand, igneous and metamorphic rocks that have lost U by recent chemical weathering may also have lost Pb. However, the isotopic ratios of the remaining Pb may not have changed if the isotopes of Pb were not fractionated. In other words, the isotope ratios of Pb in the weathered rocks are not changed if the Pb that was lost had the same isotope composition as the Pb that was present before the loss occurred. If chemical behavior is the only consideration, then perhaps this assumption could be justified; however, there are other factors such as any movement of the various Pb isotopes within the material matrix containing the Pb isotopes. Nevertheless, it is maintained that a date can be calculated based on the slope of the Pb-Pb isochron obtained from samples of even weathered rocks.
The equation for Pb-Pb isochrons is derived from combining equations (4) and (5) above to yield equation (10) above, which expresses the ratio of radiogenic 207Pb to 206Pb and then yields equation (9) above:
This is the equation for a straight line in coordinates of 206Pb/204Pb (x) and 207Pb/204Pb (y) whose slope m is
Age determinations by this Pb-Pb isochron method depend on the assumptions that all the samples that define the isochron (Faure and Mensing 2005, 241):
- had the same initial Pb isotope ratios;
- formed at the same time; and
- remained closed to U and Pb until the recent past, when they were exposed to chemical weathering.
In addition, the 238U/204Pb and 235U/204Pb ratios of the samples must have sufficient variation to allow Pb having different isotope ratios to form within them. The slope of Pb-Pb isochrons can be used for dating by solving equation (21) for t by interpolating within Table 1. Alternately, equation (21) can be solved by iteration on a computer to any desired level of precision.
The Pb-Pb isochron method has been used very widely for dating igneous and metamorphic rocks, especially those of Precambrian age, as well as meteorites. The method is claimed to yield the time elapsed since the isotopic homogenization of Pb and subsequent closure of rocks to U and its intermediate daughters. However, this ignores the known measurable leakage of the intermediate daughter Rn gas, which thus reduces the amount of in situ final 206Pb and 207Pb. Furthermore, this Pb-Pb isochron method also ignores the demonstrable fact that what are interpreted as isochron lines may instead be mixing lines between two end-member Pb isotope compositions, and there is no known way to definitively tell the difference between an isochron and a mixing line.
Common Pb Dating
Lead (Pb) is widely distributed throughout the earth, occurring not only as the radiogenic daughter of U and Th, but also forming its own minerals from which U and Th are excluded. Therefore, the isotopic composition of Pb varies between wide limits, from highly “radiogenic Pb” in supposedly old U- or Th-bearing minerals to the “common Pb” in galena (PbS) and other minerals. Pb is also a trace element in all kinds of rocks. It is claimed that its isotopic composition in rocks contains a record of the chemical environments in which the Pb resided, whether in the mantle, crustal rocks, or Pb ores. Each of those environments has different U/Pb and Th/Pb ratios that affect the isotopic “evolution” of the Pb, via magma generation and fractionation, hydrothermal and metamorphic processes, and weathering and other low-temperature processes at the earth’s surface. Furthermore, the isotopic composition of Pb in a mineral or rock may be modified both by decay of U and Th, and by mixing with Pb having different isotopic compositions. As a result, it is claimed that the isotopic compositions of Pb in minerals, rocks and ore deposits display complex patterns of variation which reflect their particular geologic histories.
The isotopic composition of this so called “common Pb” was first determined by Ashton (1927). It appeared to have a constant weight, which suggested that it might also have a constant isotopic composition. However, Nier (1938) reported systematic variations of the isotopic compositions of Pb in galenas from different sources. These leads had nearly constant atomic weights in spite of significant differences in their isotopic compositions, because increases in the 206Pb/204Pb ratios were often accompanied by comparable increases of the 208Pb/204Pb ratio. Thus the constancy of the atomic weight of common Pb is largely fortuitous. Subsequently, Nier, Thompson, and Murphy (1941) reported isotopic analyses of Pb extracted from galenas from different ore deposits. They demonstrated conclusively that such leads have variable isotopic compositions and proposed that these variations result from mixing of radiogenic Pb with “primeval” or primordial Pb prior to the formation of the galenas.
This proposal stimulated the construction of quantitative models for the supposed isotopic evolution of Pb in the earth from which the age of the earth and the age of common Pb in minerals could supposedly be determined. The first such calculation was that of Gerling (1942), who obtained an age of 3940 Myr for the earth. Then Holmes (1946) and Houtermans (1946) independently formulated a general model for Pb evolution in the earth, which has become known as the Holmes-Houtermans model. The assumptions on which this model is based are:
- originally the earth was fluid and homogeneous (a postulated necessity for naturalistic modelling, yet inherently biblical from Genesis 1:2, though it is very doubtful Holmes and Houtermans had the biblical view in mind);
- at that time U, Th, and Pb were uniformly distributed (though how do they know whether these elements even existed at that point in time?);
- the isotopic composition of the primordial Pb was everywhere the same (yet Pb is one of the heaviest metals in the periodic table, so considering the gravitational field of the earth one would not necessarily expect this to be the case);
- subsequently the earth became rigid, and small regional differences arose in the U/Pb ratio;
- in any given region, the U/Pb ratio changed only as a result of radioactive decay of U to Pb; and
- at the time of formation of a common Pb mineral, such as galena, the Pb separated from U and Th and its isotopic composition has remained constant since that time.
The Holmes-Houtermans model is thus said to account for the isotopic composition of any given sample of common Pb in terms of a single-stage history. It assumes that radiogenic Pb is produced by the decay of U and Th in the source regions and that the resulting Pb (primordial plus radiogenic) is then separated from its parents and incorporated into galenas in ore deposits. The isotopic composition of Pb in the galenas does not then change, because that mineral does not contain any U or Th.
The 206Pb/204Pb ratio of a U-bearing system of age t that has remained closed to U and all its daughters would be:
If Pb was withdrawn from such a system without isotope fractionation t years ago, then the 206Pb/204Pb ratio of that Pb would be:
This reduces to
where
(206Pb/204Pb)t = the isotope ratio of common Pb of age t,
(206Pb/204Pb)i = the isotope ratio of primordial Pb in the earth t years ago,
238U/204Pb = the ratio of these isotopes in a particular source region of common Pb in the interior of the earth at the present time,
t = time elapsed since removal of a common Pb sample from its source, and
T = age of the earth.
Of course, this factoring in equation (24) assumes that the 238U and 204Pb are the same at t and t.
Similar equations can also be written for the 235U and 232Th decay schemes. It is also helpful to introduce
| (206Pb/204Pb)i = a0 | 238U/204Pb = μ |
| (207Pb/204Pb)i = b0 | 232Th/204Pb = ω |
| (208Pb/204Pb)i = c0 | 232Th/238U = κ |
Using these symbols, the equations for the isotope ratios of common Pb according to the Holmes- Houtermans model are:
where λ3 is the decay constant of 232Th. These equations contain several constants (a0, b0, c0, μ, ω, κ and T) for which values need to be provided before these equations can be used to date samples of common Pb. However, equations (25) and (26) may be combined to eliminate μ:
This was the equation which was first used to estimate the age of the earth on the basis of the Pb isotopic compositions of galena samples of “known ages”.
In order to determine the age of the earth it was assumed that the earth condensed from colliding matter in space from the solar nebula, which is of course a totally unbiblical assumption. Meteorites are also regarded as fragments of larger bodies that similarly formed early in the history of the solar system. During the formation of their parent bodies it is conjected that the iron sulfide mineral troilite (FeS) formed. It contains appreciable concentrations of common Pb, but is virtually free of U and Th. Therefore, the isotopic composition of Pb in troilite is believed to have remained very nearly constant since crystallization. It appears to be the least radiogenic Pb available, that is, Pb containing the lowest quantities of 206Pb, 207Pb, and 208Pb. As such it is believed to be the closest representative of the isotopic composition of the earth’s primordial Pb, because the earth and the meteorites are believed to have formed at the same time from an isotopically homogeneous solar nebula. This shows how model dependent all of this dating method is, because it depends on already assuming the solar nebula hypothesis is correct.
The age of the meteorites was first established by Patterson (1956) using the isotopic composition of Pb in three chondrite and two iron meteorites. For these data he substituted t = 0 into equation (28), which thus reduces to:
This is the equation of a straight line (if t is a constant) in coordinates of 206Pb/204Pb (x) and 207Pb/204Pb (y) that passes through a point presumably representing primordial Pb whose coordinates are (a0, b0). The slope m of this line is:
Equation (30) is solved for t by interpolating in a table of values of slope m for selected values of T. It enables calculation of the age of meteorites from the slope of the line fitted to the measured values of their 206Pb/204Pb and 207Pb/204Pb ratios. Patterson’s (1956) data for three chondrite and two iron meteorites in Fig. 6 fit a line whose slope is 0.6022, which corresponds to a date of t = 4.55 ± 0.07 Ga based on the decay constants he used, though it is hard to believe this level of accuracy was possible when Patterson made the measurements. In the Patterson (1956) paper he only mentions 2% and 1% as possible errors, then quotes 4.55 ± 0.07 Ga as the final error for his age estimate. Questions about the level of accuracy of his measurements would be better resolved if he had provided his measurements of the U, Pb, and Pb isotope concentrations, instead of just the Pb isotope ratios.
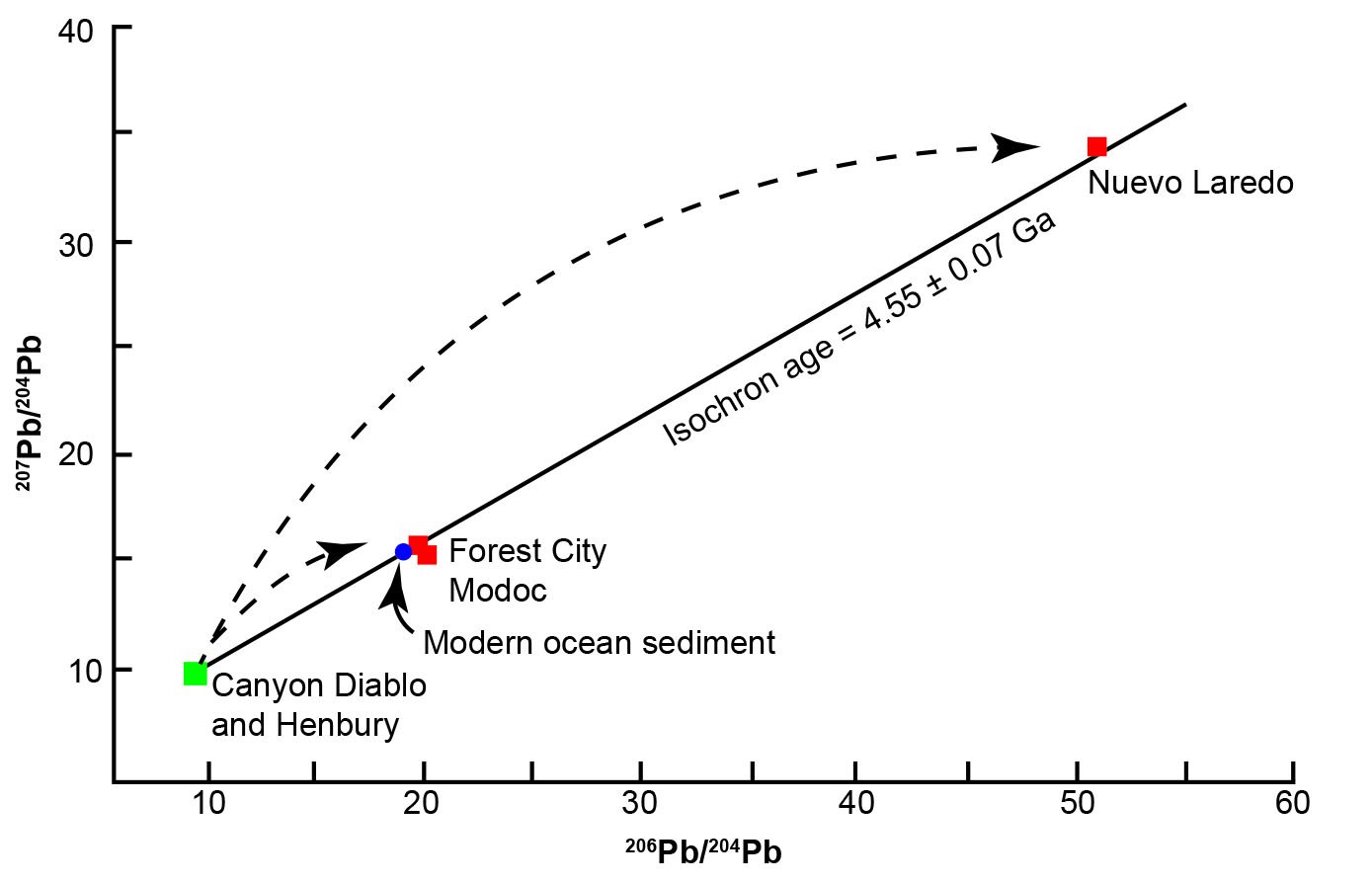
Fig. 6. Lead isochron for meteorites and modern ocean sediment that yielded from its slope the age of the earth as 4.55 Ga and is called the geochron (after Patterson 1956).
Patterson (1956) also evaluated the assumption that the age of the earth is similar to that of meteorites. He reasoned that if meteorites and the earth have a common age, and if they initially contained Pb of the same isotopic composition, then average terrestrial Pb would lie on the line formed by the meteorites. However, this begs the question as to why should the earth and the meteorites have a common age and initially contain Pb of the same isotopic composition, which is based solely on the unbiblical assumption that the earth and the asteroids which parented the meteorites formed out of the solar nebula. Nevertheless, Patterson (1956) chose Pb from recent oceanic sediment, because he argued it was a representative sample of terrestrial Pb, and showed that it fitted the meteorite Pb-Pb isochron line within experimental errors (fig. 6). However, deep sea sediments contain Pb whose isotopic composition varies regionally and not all of them fit the meteorite isochron as well as the sample chosen by Patterson. Nevertheless, the demonstration by Patterson of the similarity of modern terrestrial and meteoritic Pb seems to support the conclusion that the age of the earth is essentially the same as that of the meteorites, and that the isotopic composition of the earth’s primordial Pb could be closely approximated by the Pb in meteoritic troilite. The values for the isotopic ratios of this primordial Pb were first reported by Tatsumoto, Knight, and Allègre (1973) who analyzed Pb in troilite from the Canyon Diablo iron meteorite, and then confirmed by Chen and Wasserburg (1983) (table 3).
|
|
|
Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9.307 | 10.294 | 29.476 | Tasumoto, Knight, and Allègre (1973) | |||||||||
| 9.3066 | 10.293 | 29.475 | Chen and Wasserburg (1983) |
The equations (25)–(28) describe the Holmes- Houtermans model and enable the determination of the ages of common leads that have single-stage histories. The model assumes that all samples of common Pb are mixtures of radiogenic Pb, which formed in closed source regions having differing values of μ and ω, with primordial Pb. Equations (25) and (26) can be used to calculate the 206Pb/204Pb and 207Pb/204Pb ratios of Pb removed at different times t from source regions that have specified values of μ. A large number of ore deposits contain common Pb that seems to have developed in systems having μ values greater than 8, but less than 10. Accordingly, Fig. 7 shows three Pb growth curves for μ = 8, 9, and 10, assuming the age of the earth t is 4.55 Ga.
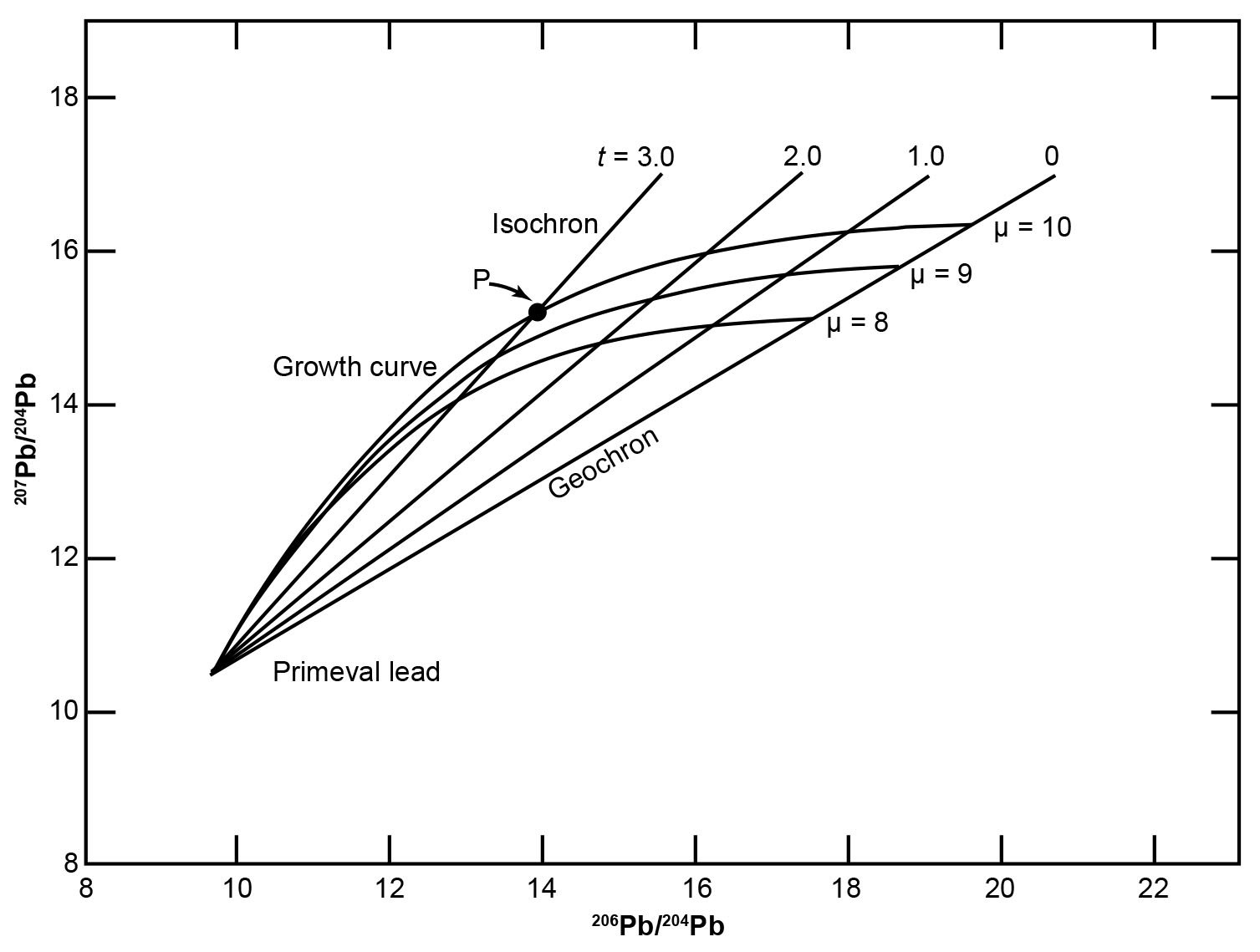
Fig. 7. Graphical representation of the Holmes-Houtermans model (after Faure and Mensing 2005). The curved lines are Pb growth curves for U-Pb systems having present day μ values of 8, 9, and 10. The straight lines are isochrons for selected values of t. This diagram was constructed by solving equations (24) and (25) assuming the age of the earth is 4.55 Ga.
Fig. 7 shows the Pb growth lines form a fan-shaped array of curved trajectories that spread out from the point representing the supposed primordial Pb. The position of a point on a particular growth curve is said to indicate the time t when that Pb was removed from its source region and was subsequently deposited in the earth’s crust as a common Pb mineral such as galena. For example, the coordinates of the point P are the isotopic ratios of a common Pb that apparently developed in a source region having a value of μ = 10 that was removed from its source at 3.0 Ga. This is termed the “model date” of the sample, which is regarded as a close upper limit to the age of the ore deposit from which the sample was taken.
It should be apparent that to interpret the isotope ratios of common Pb in the Holmes-Houtermans model both μ and t need to be determined. This is possible because from the two independent equations (25) and (26) the compatible values of the two unknown variables can be determined. However, those two equations were combined into equation (28) to eliminate μ as a variable. When t is constant, equation (28) reduces to a family of straight lines, all of which pass through a common point supposedly representing primordial Pb (fig. 7). The slopes of these lines are:
They depend only on t, provided t is known, that is, the assumed age of the earth as determined by Patterson (1956). All single-stage leads that were removed from their sources at the same time t must lie on these straight lines, which are isochrons, because all single-stage leads that lie on a particular line are defined as having the same age. Common leads that grew in different source regions and were removed from them at the same time plot at the intersections of their respective growth curves with the isochron corresponding to their presumed age. A series of such isochrons representing different values of t have been drawn in Fig. 7. The isochron representing leads having t = 0 is called the geochron, because all modern single-stage leads in the earth and in meteorites supposedly lie along it and the slope represents the age of the earth as determined by Patterson (1956).
Equation (28) obviously cannot be solved by conventional algebraic methods to calculate the model date of a sample of common Pb because it is transcendental. Instead, it is solved to any desired level of accuracy by a graphical method, or by means of a table giving the slopes of isochrons as a function of t (table 4). The slope of the isochron on which a particular Pb must lie (assuming it had a single-stage history, is derived from equation (28), each side of which is equal to m. Its measured 206Pb/204Pb and 207Pb/204Pb ratios and the a0 and b0 values from Tatsumoto, Knight, and Allègre (1973) are substituted into the left-hand side of equation (28) to obtain the slope, and then that slope corresponds to an age interpolated from the values listed in Table 4. After the model date has been calculated, it can be substituted into equation (25) to solve for μ.
| Age (t) Ga | Slope (m) |
|---|---|
| 0 | 0.61761 |
| 0.2 | 0.63705 |
| 0.4 | 0.65509 |
| 0.6 | 0.67624 |
| 0.8 | 0.69923 |
| 1.0 | 0.72426 |
| 1.2 | 0.75158 |
| 1.4 | 0.78144 |
| 1.6 | 0.81415 |
| 1.8 | 0.85005 |
| 2.0 | 0.88952 |
| 2.2 | 0.93300 |
| 2.4 | 0.98101 |
| 2.6 | 1.03410 |
| 2.8 | 1.09295 |
| 3.0 | 1.15830 |
| 3.2 | 1.23100 |
| 3.4 | 1.31205 |
| 3.6 | 1.40257 |
| 3.8 | 1.50387 |
| 4.0 | 1.61743 |
| 4.2 | 1.74498 |
| 4.4 | 1.88849 |
| 4.6 | 2.05025 |
|
λ1 = 1.55125 × 10-10y-1, λ2 = 9.8485 ×10-10y-1, T = 4.55 ×109 y |
|
The geological validity of the model date and the μ value of the source region depend on the assumption that the Pb actually had a single-stage history. That assumption must be tested before the model date could be used to signify a presumed age of the ore deposit from which the sample of common Pb was taken. The criteria that need to be used for that assessment include (Faure and Mensing 2005):
- the model dates of a representative suite of samples from a given deposit must be concordant, unless there is evidence of episodic mineralization spanning an interval of time;
- the isotope ratios of Pb from a given deposit must be constant within experimental error;
- the model dates must be positive numbers; and
- the model dates should be in general agreement with isotopic dates of other minerals from the ore and country rock.
However, exact agreement is not to be expected because the model common Pb date reflects a different event than the isotopic dates of the silicate minerals.
Ironically, the number of ore deposits that meet these stringent requirements of the single-stage model is quite small. Kanasewich (1968) identified only ten such deposits (out of the hundreds known at that time) containing so-called “ordinary” leads that lie along a single growth curve. Stanton and Russell (1959) recognized that ore deposits containing such apparent single-stage leads occur in stratigraphic sequences of volcanic and sedimentary rocks of supposedly marine origin in volcanic island arcs (which in the biblical model of earth history were produced during the global Flood cataclysm). Thus, the Pb in these conformable deposits was thought to have been derived from lower crustal and mantle sources and was emplaced by volcanic activity without contamination by radiogenic Pb from the crust. However, most of the world’s ores contain leads that yield erroneous dates by the single-stage model and are therefore classified as “anomalous.” Some anomalous leads actually give negative dates that lie in the future because they contain more radiogenic Pb than is compatible with the single-stage model. Thus, this presence of apparently excess radiogenic Pb in many ore deposits is a clue that the U/Pb ratio of the sources of ore leads has probably increased with time either continuously or episodically.
Therefore, Stacey and Kramers (1975) developed a two-stage model in which the evolution of Pb started with primordial isotope ratios at 4.57 Ga (table 5 and fig. 8). At a more recent date, the U/Pb ratio of the reservoir was changed by geochemical differentiation, and then remained constant to the present. In order to construct this model Stacey and Kramers specified the average crustal 206Pb/204Pb, 207Pb/204Pb, and 208Pb/204Pb ratios with values derived from the average isotope compositions of Pb in sedimentary and volcanic rocks deposited in the oceans, and from Pb-Pb isochrons of ancient granitic rocks that intersect each other in a common point, presumably because that point represents the average crustal Pb. This is ostensibly so because the expected value of average crustal Pb would be the average of the basaltic oceanic crust, the granitic continental crust, and the sedimentary veneer on both, weighted according to their respective volumes. They also used the Pb isotope ratios from 13 conformable ore deposits.
| Time, Ga |
|
|
|
|
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start, Stage 1 | 4.57 | 9.307 | 10.294 | 29.476 | 7.192 | 32.208 | ||||||||||||
| Start, Stage 2 | 3.70 | 11.152 | 12.998 | 31.230 | 9.735 | 36.837 | ||||||||||||
| Present | 0 | 18.700 | 15.628 | 38.630 | 9.735 | 36.837 |
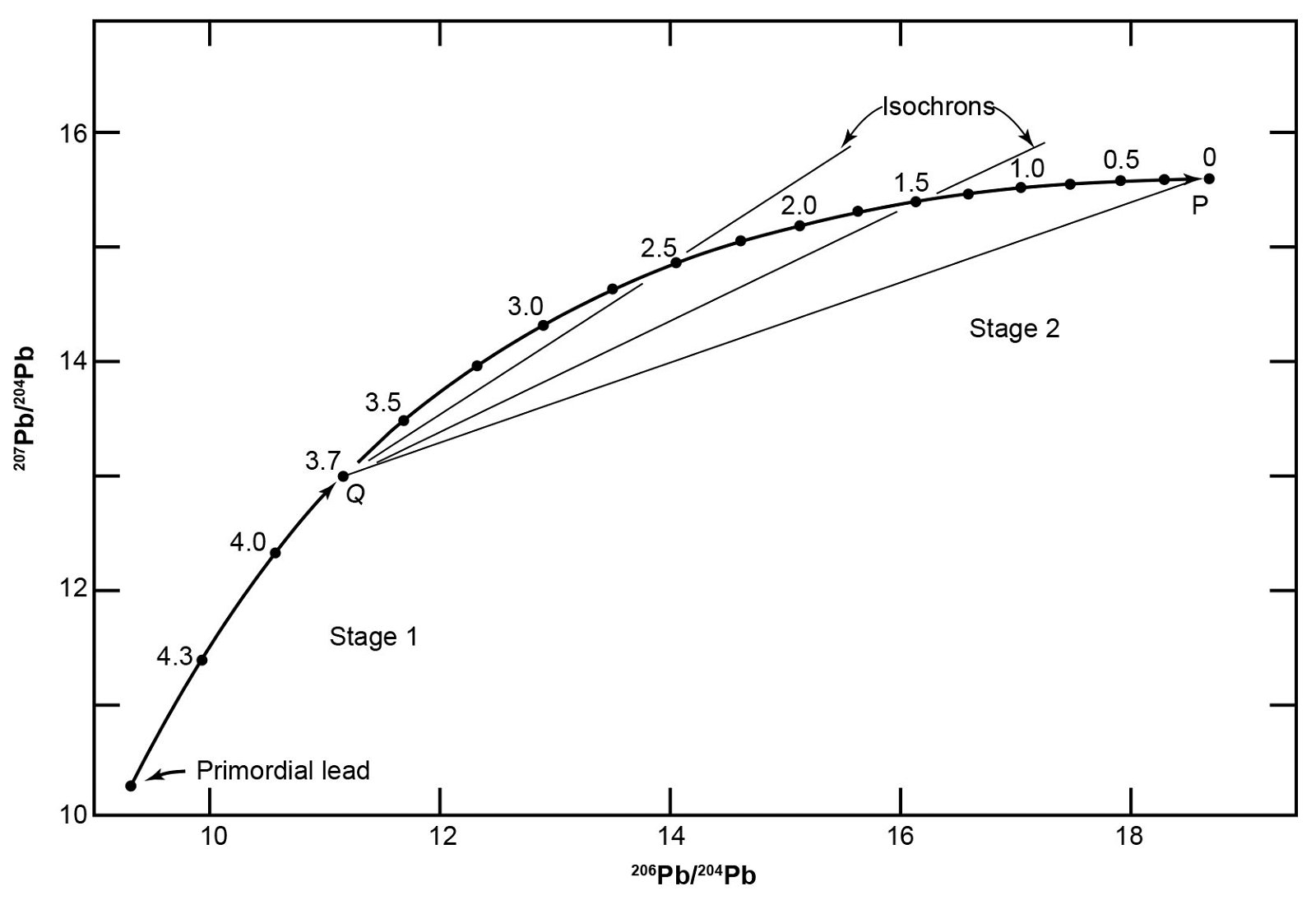
Fig. 8. Two-stage Pb evolution model of Stacey and Kramers (1975) summarized also in table 5 (after Faure and Mensing 2005). In this model Pb evolves from primordial ratios between 4.57 Ga and 3.70 Ga in a reservoir with 238U/204Pb = 7.192. At point Q (t = 3.70 Ga) on the evolution line the 238U/204Pb ratio of the reservoir was changed by chemical differentiation to 9.735. Pb evolution then continued undisturbed to point P representing average crustal Pb. Straight lines connecting any point on the evolution line between 3.70 Ga and the Present to Q are isochrons. The slopes of such isochrons are related by equations to the time elapsed since a Pb sample was isolated from the reservoir.
In the Stacey-Kramers model Pb evolved between 4.57 Ga and 3.70 Ga in a reservoir having uniform 238U/204Pb and 232Th/204Pb ratios (table 5 and fig. 8). At 3.70 Ga the values of the 238U/204Pb and 232Th/204Pb ratios changed by chemical differentiation, and subsequently the reservoir remained undisturbed until the present. All leads that evolved in such a reservoir, and were removed from it at some time in the past, must have isotope ratios that lie on the growth curve between 3.70 Ga and the present. The time of separation from the reservoir can supposedly be calculated using the isochron that starts at 3.70 Ga and cuts the growth curve between then and the present.
It needs to be noted that it is not clear where the Pb reservoirs are actually located within the earth. It had been concluded that the Pb reservoir was in the mantle, because it was believed that it is sufficiently homogeneous to permit the ordinary leads of conformable ore deposits to evolve. However, measurements by Gast, Tilton, and Hedge (1964) of the isotopic composition of Pb in young volcanic rocks derived from the mantle soon demonstrated that idea is untenable (Richards 1971). Instead, it appears that ore leads may have originated in sediments that were homogenized by repeated cycles of erosion, transport, and deposition before they were finally melted in subduction zones. Some of the Pb may have been extracted from the resulting magmas in the course of volcanic activity as conformable ore deposits associated with interbedded volcanic and sedimentary rocks. In addition, Pb may have been transported by hydrothermal fluids from cooling plutons for deposition of ore deposits within them, or transported out into the country rocks for deposition of vein and replacement ore deposits. While Pb is insoluble in water, hydrothermal fluids contain high concentrations of Cl and S which make Pb soluble in them. The Pb in such disconformable ore deposits is susceptible to contamination by mixing with other leads during transport, or by association with U- and Th-bearing minerals after deposition. For this reason, it appears that leads in disconformable ore deposits frequently have variable isotope ratios that define straight lines in the Pb isotope evolution diagrams of both single-stage and two-stage models. Such linear arrays of isotope ratios can seemingly be interpreted regardless of whether the one- or two-stage model is used, provided they were caused by the addition only of radiogenic Pb and not by mixing of two common leads with different isotopic compositions.
However, in spite of the efforts to improve so-called Pb-evolution models (for example, Doe and Zartman 1979; Zartman and Haines 1988), it was in large part the discovery that modern lavas, particularly oceanic basalts, yielded old radioisotope “ages” which led to the recognition and definition of geochemical reservoirs in the mantle where these lavas had been sourced (Snelling 2000). Zindler and Hart (1986) delineated five end-member compositions in the mantle by which a variety of mixing processes were regarded as capable of explaining all the Sr-Nd-Pb isotope geochemical data pertaining to mid-ocean ridge and ocean-island basalts around the globe. Similarly, Taylor, Jones, and Moorbath (1984) had recognized three isotopic reservoirs in the continental crust, also characterized with respect to Sr-Nd-Pb isotopes. What these mantle and crustal isotopic/geochemical reservoirs (whose isotopic characteristics were listed by Rollinson 1993) actually represent is still somewhat uncertain and the subject of ongoing investigations. But these reservoirs and their isotopic compositions have been linked in mantle-crust dynamics models to the processes of plate tectonics through earth history in order to solve what had become known as the “Pb isotope paradox” (for example, Albarède1998; Brandenburg et al. 2008; Castillo 2016; Doe and Zartman 1979; Kramers and Tolstikhin 1997; Kumari, Paul, and Stracke 2016; Murphy, Kamber, and Collerson 2003; Phipps Morgan, and Morgan 1999; van Keken, Hauri, and Ballentine 2002; White 2015; Zartman and Haines 1988; Zindler and Hart 1986). It is envisaged that complex mixing has occurred through time as the upper and lower mantle have been stirred by the subduction of plates, convection, and the ascent of plumes. Such whole-mantle convection is also envisaged in the catastrophic plate tectonics model for the global Flood cataclysm (Austin et al. 1994), and the discovery of former oceanic crustal plates still relatively cold at the base of the mantle is confirmation of their subduction right through the mantle. Crustal growth has thus resulted, and has also involved mixing of the various crustal isotopic reservoirs.
Defining Errors and Uncertainties
Before investigating further how the geochronology community handles the determination of, and correction for, common or initial Pb, it is necessary to define a few terms and discuss how they are used. These are the terms “error” and “uncertainty,” “accuracy” and “precision.” They are used frequently in all discussions on the methodology of U-Pb geochronology, as the dating results from the various analytical equipment are reported and contrasted. The goal of the geochronology community is understandably to reduce errors and uncertainties to produce better accuracy and precision. The fact that after a decade or more of intense intentional effort little progress has been made. The sources of error are still not “tamed” and “uncertainties” adequately reduced is testimony to the severe problems that still plague this U-Pb dating method.
Schoene et al. (2013) described the use of the terms “accuracy,” “precision,” “error,” and “uncertainty” in U-Pb geochronology, and differentiating between these terms and defining them is very useful for discussing data measurement and reporting protocols. Potts (2012) utilized the International Vocabulary of Metrology guidelines and defined “measurement error” as a “measured quantity value minus a reference quantity value.” This automatically introduces bias into the whole process because the reference value also must be previously measured with its own measurement error. Potts (2012) also defined the “measurement uncertainty” as a “non-negative parameter characterizing the dispersion of the quantity values being attributed to a measurand [defined as the quantity intended to be measured], based on the information used.” Thus, an error is a single value (for example, 0.1) and is not known unless a reference value exists to compare against. In contrast, an uncertainty is a range (for example, 99.9 ± 0.1) that is expected to contain the true value with a given probability, often referred to as a confidence interval.
Measurement error can be random (unpredictably offset from the measurand value) or systematic (consistently or predictably offset from a reference value) (Potts 2012). Once quantified, a systematic error is referred to as a bias. Each type of error has an uncertainty associated with it. These uncertainties are commonly referred to as systematic or random in reference to the error to which they relate. The uncertainty related to random error is reflected in the measurement precision (“closeness of agreement between indications or measured quantity values obtained by replicate measurements on the same or similar objects under specified conditions” [Potts 2012]), and can be reduced by increasing the number of measurements. This standard deviation of the mean (historically referred to as “standard error”) represents the confidence in the determined average value, but does not reduce the actual scatter in the data (that is, the standard deviation of the population remains the same). The uncertainty related to a systematic error reflects how well that bias can be quantified when determined under differing conditions, that is, how reproducibly it can be measured; it cannot be further reduced simply by acquiring more measurements. The use of the term “error” should be reserved exclusively for its defined purpose—to refer to the offset of the measurement from a mean or expected value. When referring to a confidence interval, the term “uncertainty” should always be used.
Of course, the expected value is fundamentally a theoretical value deduced from application of current theories and/or laws of science and it may or may not be accurate. Thus the purpose of the measurements is to confirm or deny the predictions/expectations of theory. In this context of U-Pb radioisotope dating of rocks and minerals the expected value is very much determined by theory, that is, where the rocks fit within the presumed geologic record and its timescale based on evolutionary assumptions. Yet any number of other factors, such as the initial composition, inheritance when subsequently formed, contamination after formation, and accelerated nuclear decay during a past catastrophic geologic event, could all differently influence the expected value and hence the uncertainty in what the true age might be.
Although it is common practice for geochemists to express uncertainties using σ, strictly this should be reserved only for statistics relating to a total population (the population standard deviation). Since geochemists only ever take a representative sample of a population (rather than analyzing an entire granite pluton or, in this example, every zircon within it), the correct statistical term is s (the sample standard deviation). However, both terms explicitly refer to the “standard deviation,” while the reported uncertainties derive from the standard error of the mean of isotope ratios, combined often with a statistical analog of a standard deviation (the excess variance parameter as described). These are ultimately combined with several other systematic uncertainties—for example, from TIMS measurements of reference materials and from the isotopic purity and particle-counting experiments that determine decay constants. Indeed, particle-counting experiments are usually dominated by the random/statistical errors of the measurement, especially in situations involving very low count rates such as is the situation for long-lived radioisotopes such as U and Th. The result is neither a standard deviation nor a standard error, but an uncertainty which describes the dispersion of the resulting normal distribution.
The Problem of Initial (or Common) Pb or Primordial Pb
Among the potential problems which cause possible inaccuracies in obtaining reliable U-Pb and Pb-Pb ages is the one listed by Amelin et al. (2009) as the “presence of non-radiogenic Pb of unknown isotopic composition,” which they commented is “the most important and common problem of all” that is “recognized by most of the (geochronology) community.” The non-radiogenic Pb they refer to is that Pb incorporated into the rock or mineral being dated when it formed, which did not derive from the subsequent in situ decay of U and Th. Such common or initial Pb is considered to consist primarily of 204Pb, which is the stable Pb isotope not derived by parent radioisotope decay. However, common or initial Pb invariably also contains some 206Pb, 207Pb, and 208Pb. The challenge then for the geochronologist is to determine how much 206Pb, 207Pb, and 208Pb was in the initial “common” Pb incorporated in the rock or mineral when it formed, and how much 206Pb, 207Pb, and 208Pb is due to subsequent in situ decay of U and Th. The reality is that this most important and common problem of all is without doubt a consequence of the first of the several assumptions involved in the various U-Pb and Pb-Pb model and isochron dating methods, which is that the starting conditions can be known. That assumption, like the others, has a somewhat tenuous validity because it is based on an unknown and unconfirmed uniformitarian evolutionary history for the origin and formation of the earth. This again illustrates how one’s worldview affects the interpretation of the data.
The incorporation of common or initial Pb in zircons
Of the several minerals now routinely used in U-Pb chronology, zircon is by far the mineral of choice. Indeed, it is usually claimed that zircon grains make excellent U-Pb geochronometers. This is because it is claimed that when they crystallize they do not incorporate Pb atoms into their crystal lattice structure. Thus it is presumed that all the Pb measured in them today has been added by radiodecay of parent U and Th atoms since the grains crystallized. This is because the prevalent Pb2+ atoms have too large an ionic radius (1.32 Å) and the wrong ionic charge to substitute for Zr4+ atoms (ionic radius of 0.84 Å) in the zircon crystal lattice (Watson et al. 1997). However, Pb4+ atoms have an ionic radius of only 0.91 Å and the right ionic charge, so like U4+ atoms with an ionic radius of 1.00 Å, Pb4+ atoms can substitute for Zr4+ atoms in the zircon crystal lattice. Nevertheless, since the stable form of Pb at the igneous and metamorphic conditions appropriate to zircon growth is either Pb0 or Pb2+, it is not surprising that Pb exhibits broadly incompatible behaviour toward zircon (Watson et al. 1997). On the other hand, it cannot be assumed that Pb2+ is totally excluded during growth either, because zircon is known to accommodate some altervalent substitutions to quite high concentrations, even without charge balancing cations (for example, 1.2 wt.% Dy3+ for Zr4+). So the reality is, as recognized by Amelin et al. (2009), some non-radiogenic Pb of unknown isotopic composition will always be present in zircons and other minerals being U-Pb radioisotope dated. This is the most important and common problem of what is known as “common Pb” (initial, or even some primordial Pb).
Experiments done by Watson et al. (1997) to test the incorporation of Pb into growing zircon crystals produced results that overall are consistent with the low but variable levels of non-radiogenic (common) Pb routinely found in natural zircons. They found that for zircons grown from a high-temperature SiO2-ZrO2 melt with 66 wt.% PbO in it only <1 ppm Pb was incorporated in them. On the other hand, when ~5 wt.% P2O5 was added to the melt the uptake in the zircons increased to ~1500 ppm Pb. In the case of natural zircons immersed in zircon-saturated hydrothermal solutions containing either PbO2 or PbO plus P2O5, the resulting zircon overgrowths contained > 3 atom% Pb, with apparent zircon/fluid partition coefficients of 4.2 and 2.6, respectively, for Pb4+ and Pb2+. Yet in the resulting zircon overgrowths P is absent, suggesting that the charge balance is accomplished by H+ instead. In further experiments the small zircons grown by cooling aqueous solutions (PbO + SiO2 + ZrO2 ± P2O5) from 800°C or 900°C contained ~2–4 wt.% PbO, yielding apparent partition coefficient values of ~0.2–0.3. Available P5+ was also incorporated in a 2:1 ratio with Pb2+. However, Pb entered these zircons even when P was unavailable, so it was again concluded that H+ plays a charge-balancing role. Watson et al. (1997) thus concluded that because of the rapid, polythermal modes of zircon growth and the high Pb content of their experimental systems, the apparent partition coefficients should only be viewed as qualitative indicators of Pb compatibility under various growth circumstances. Yet their overall results are consistent with low but variable levels of non-radiogenic (common) Pb in natural zircons.
Ireland and Williams (2003) confirmed that H+ plays a charge-balancing role with Pb in zircons by showing that PbH+ (lead hydride) is present in them. They also admitted that the presence of common Pb in zircon analyses by secondary ion mass spectrometry (SIMS) cannot be ignored if accurate U-Pb ages are to be calculated. Indeed, apart from common Pb incorporated in zircons from their host rock environments at the time of their formation, common Pb in zircons can originate from several other sources—sub-microscopic mineral inclusions, Pb added to the zircons during or after alteration, Pb physically trapped in microfractures, and laboratory Pb from polishing compounds, aerosols, and coating materials. Each has a different composition, so the measured common Pb is a mixture. Ireland and Williams (2003) maintained that in practice, low levels of common Pb are assumed to be laboratory-derived, and higher levels to be mixtures of laboratory Pb and rock Pb.
Determining the amount of common or initial Pb using TIMS
Bowring and Schmitz (2003) also reported that all zircon analyses have a component of common Pb. There has been some controversy historically over just how much common Pb is incorporated into the zircon crystal structure and how much occurs along fractures, in solid and fluid inclusions, and in analytical blanks. Thus, they claimed experimental work has demonstrated that most zircons contain little to no indigenous common Pb at the pictogram level, and has determined the limited solubility of Pb in the crystalline zircon structure. Furthermore, in situ sensitive high resolution ion micro probe (SHRIMP) analyses have further demonstrated the vanishingly small amounts of common Pb in the majority of zircons, as claimed to have been determined by the methods elaborated on below. Thus, although there are documented exceptions, including poorly understood incorporation into radiation-damaged zircons (Mattinson 1994), most common Pb in isotope dilution thermal ionisation mass spectrometer (ID-TIMS) analyses is claimed to be apparently hosted by inclusions, present as surface contamination, or introduced during chemical processing.
As a result, minimization of laboratory blanks has remained the single most important requirement for high-precision U-Pb analyses, so most laboratories now claim to have reduced analytical blanks to below 5 picograms, and some to less than 1 picogram, of Pb. And now that it is claimed that diamagnetic, clear, crack- and inclusion-free zircons separated from volcanic rocks have little to no indigenous common Pb, the assumption that all measured common Pb arises from laboratory blanks appears warranted for the many samples with picogram levels of total common Pb. Thus most laboratories incorporate a substantial uncertainty in the magnitude of their laboratory blank into error propagation, which is usually the dominant source of error in each analysis (Ludwig 1980). However, the isotopic composition of laboratory blank Pb is also of concern. Common Pb in laboratories is in airborne particulates, labware, and reagents, and the contributions from these sources can change over time. Thus most laboratories have characterized and adopted an isotopic composition with a realistically large uncertainty for their blank that includes temporal variability.
Bowring and Schmitz (2003) thus concluded that even in the best geochronology laboratories the total amount of common Pb in an analysis can exceed estimated laboratory blanks. Thus the assignment of an isotopic composition to this supposed indigenous initial or common Pb can have a discernible effect on the calculated date and error associated with an analysis, depending on the contrast between the assumed blank and the initial Pb isotopic compositions. Therefore, most geochronology laboratories use two approaches for estimating the composition of initial or common Pb. The simplest is to use a model for Pb isotopic evolution such as that of Stacey and Kramers (1975) and assign a model composition corresponding to the nominal age of the zircon being analyzed. Yet while that model was proposed to describe the average evolution of Pb in a mantle reservoir, many silicic, zircon-bearing rocks are derived from melting older crust rather than mantle, thus calling into question the applicability of this model approach. Another approach has been to use the isotopic composition of Pb in a comagmatic low U/Pb mineral such as alkali feldspar (Housh and Bowring 1991). However, in general, the older the rocks the less likely it is that fresh feldspar can be recovered from them and that its isotopic composition can be determined.
Bowring and Schmitz (2003) also admitted that the uncertainty in the composition of the initial or common Pb is not usually propagated into the age error for many zircon geochronological applications, because it is believed its contribution is minimal in radiogenic samples. However, for high-precision geochronology and calibration of the geological timescale, where a complete description of uncertainties is vital to the interpretations, they maintained that the best procedure is for this systematic error to be propagated by calculating individual analysis dates and errors using reasonable bounds on initial or common Pb compositions, deriving weighted means and errors for the resulting data sets, and appropriately supplementing the weighted mean error calculated from the assumed average initial or common Pb composition according to the resultant dispersion introduced by varying the common Pb composition. However, they emphasized that in any zircon U-Pb analysis the most crucial parameter is the ratio of radiogenic to common Pb, often indirectly expressed by the measured 206Pb/204Pb ratio. The higher this ratio the less sensitive it is believed the analysis is to the content and compositions of both the blank and initial or common Pb. Thus, ultimately analyses with low radiogenic to common Pb ratios are claimed to be best rejected or given less weight in the age calculation, as the incorrect assignment of common Pb and uncertainty can have a discernible effect on the calculated age. This is especially true for analyses of young, low-U zircons where the radiogenic to common Pb ratio can approach unity (Bowring et al. 1998; Mundil et al. 2001). In other words, samples that are considered too young are automatically rejected.
Schmitz and Schoene (2007) provided a comprehensive treatment of the derivation of U-Pb isotope ratios and their corresponding uncertainties from ID-TIMS measurements in high-precision geochronology. Standard parametric statistical methods of error propagation are utilized to convolve uncertainties associated with the various sources of error, including the blank Pb subtraction and the initial or common Pb correction. Thus they propagated the amount and isotopic composition of blank Pb contributions and the initial or common Pb in each analyzed mineral grain, the initial or common Pb being defined as the Pb incorporated into the growing crystal at the time of its formation. Because the amount of both 204Pb and 206Pb measured as initial or common Pb is subtracted from each analysis is sample dependent, Schmitz and Schoene (2007) considered the errors for each should be propagated on a sample-by-sample basis. However, they did advocate that to develop a firm estimation of the sensitivity of a data set to the assumed initial common Pb component, the constituent data be reduced with a range of geologically reasonable initial Pb isotope ratios, or better a full Monte Carlo simulation of initial Pb isotope ratios, and the variance in the resulting radiogenic model ages be then incorporated into the final age interpretation (Schmitz and Bowring 2001). By “geologically reasonable initial Pb isotope ratios” they of course mean assumption driven selection of suitable standards that produce the desired results based on their evolutionary geology model, because there are no objective absolute standards for initial Pb isotope ratios.
After performing the error propagation calculations to derive the uncertainty in the sample (initial) 204Pb, Schmitz and Schoene (2007) assessed the contributions of the errors associated with the blank and initial or common Pb subtraction. They found that due to the nature of the partial derivatives of radiogenic 206Pb with respect to the Pb blank amount and the blank and initial Pb composition, the error contributions from these three variables are relatively minor in zircon analyses. Of the three quantities, the Pb blank amount predominates over a wide range of 206Pb/204Pb, yet for the assumed blank amount in the range of 1–2 picograms the contribution to the 206Pb*/238U error from the blank amount uncertainty (being negatively correlated with 206Pb/204Pb) only exceeds 10% at 206Pb/204Pb < 1000. They also found that zircon analyses with substantially lower contributions to the 206Pb*/238U error from the blank amount uncertainty have significantly lower total common Pb and assumed blank amount uncertainty, the average common Pb being ~0.3 picogram, and all common Pb is assumed to be in the blank. This would appear to illustrate how modern low-blank analysis can substantially mitigate errors associated with the common Pb correction. Schmitz and Schoene (2007) thus suggested that if procedural blanks were reduced below 0.5 picogram, then the analysis of only 5 picogram of 206Pb* would apparently be required to essentially obviate errors associated with the common Pb correction. They also noted that the blank and initial common Pb composition uncertainties usually have contributions to the 206Pb*/238U error nearly two orders of magnitude less than that contributed from the Pb blank amount. However, an interesting crossover in error contributions apparently occurs at 206Pb/204Pb ratios of approximately 100–200, whereby the error contributions from uncertainty in the initial or common Pb composition begin to predominate over not only the other common Pb variables, but all sources of error.
Schaltegger, Schmitt, and Horstwood (2015) emphasized that zircon analyses by ID-TIMS may contain minute amounts of two types of Pb that are unrelated to U and Th decay. The first is initial non-radiogenic Pb, commonly referred to as “common-Pb,” which is incorporated during crystallization. Radiogenic Pb (Pbr) is subsequently added to the common Pb by U radiodecay. The uncertainty in the isotopic composition of the common Pb correction (Pbc) dominates the analytical uncertainty at low Pbr/Pbc, and mainly affects the 207Pb/235U system. They also maintained that the isotopic composition of Pbc may be estimated from the crustal growth Pb-evolution model of Stacey and Kramers (1975) or from analysis of U-deficient minerals in the same sample, such as feldspars. Since most analyzed zircons appear to contain very limited amounts of Pbc, the Pbr/Pbc is mainly limited by the procedural blank. The second type of Pb is the procedural blank Pb, which is introduced during chemical separation and analysis, and can apparently be distinguished from common Pb by its different isotopic composition. Schaltegger, Schmitt, and Horstwood (2015) maintained that state-of-the art procedures may yield blank levels as low as 0.2 picogram, including from dissolution, ion chromatography and isotope analysis. Such low blanks are a prerequisite to analyze small, low-U and/or young zircons at high precision. Thus, accurate knowledge of the isotopic composition of the procedural blank component is essential at Pbr/Pbc ratios < 5, as the uncertainty on the isotope ratios used must be propagated onto the final U-Pb dating result. Thus, Schaltegger, Schmitt, and Horstwood (2015) concluded that ultimately the accuracy of a U-Pb date determined by ID-TIMS is mainly determined by the accuracy of the tracer calibration, as well as by the U decay constant uncertainty.
Determining the amount of common or initial Pb using SIMS
So how is common Pb measured in zircons when the 206Pb and 207Pb atoms of common or initial Pb are identical to the 206Pb and 207Pb atoms produced in situ by radiodecay? Ireland and Williams (2003) discussed several different ways the common Pb content can be estimated when using SIMS technology for zircon geochronology. They maintained that the most direct method is by measuring 204Pb, the non-radiogenic Pb isotope that is thus unique to common Pb. Then by knowing the initial Pb isotopic composition, the other Pb isotopes can be subtracted from the analysis. So if f is defined as the fraction of total 206Pb in an analysis that is initial 206Pb, that is,
then f can be calculated from the measured 204Pb/206Pb as
assuming the ratio (204Pb/206Pb)tot is always a constant throughout time. However, Ireland and Williams (2003) also maintained that the isotopic composition of initial rock Pb can be measured on cogenetic common Pb-rich minerals such as feldspar, or estimated from common Pb growth curves, knowing the approximate age of the zircon whose age is being determined. The latter procedure involves circular reasoning. The composition of laboratory-derived Pb may already be known based on the laboratory’s physical location, assuming it never changes over time. For example, in continental Australia it is that of late Proterozoic massive Pb sulfide ore. Alternately, if a cogenetic suite of zircon grains has a wide range of common Pb contents, the composition of the common Pb can apparently be estimated with reasonable reliability by plotting a set of Pb isotope and U/Pb mixing lines and extrapolating back to zero U.
Although Ireland and Williams (2003) maintained that 204Pb provides the most direct measure of common Pb, they realized that the low relative abundance of 204Pb can make that correction procedure imprecise, particularly for young or low-U zircons with low 207Pb/204Pb. So they suggested a more precise estimate of the common Pb can sometimes be made from the 208Pb/206Pb and the measured Th/U ratios, f being calculated as:
To calculate the expected radiogenic 208Pb/206Pb from the Th/U ratio relies on the assumptions that neither the Th/U nor radiogenic 208Pb/206Pb ratio has changed throughout the zircon’s history, except by radioactive decay, and that the zircon’s age is known. The last requirement is said not to be critical, as the factor relating radiogenic 208Pb/206Pb to Th/U ranges only from 0.25 to 0.32 over the full range of postulated geological time. Ireland and Williams (2003) maintained that this correction method normally works very well for zircon with low Th/U (<0.1), but becomes less precise for Th-rich zircon (Th/U >1) as radiogenic 208Pb/206Pb approaches the 208Pb/206Pb of the common Pb. It is also prone to error, because altered zircon tends to lose 208Pb more readily than 206Pb, resulting in an underestimate of the common Pb content. A variant of this procedure devised by Andersen (2002) seems to take better account of Pb loss, but requires the additional assumption that the time of the Pb loss is known.
Ireland and Williams (2003) also suggested that if it is assumed the zircon analyses are concordant, that is, the zircon’s U-Pb ages are in agreement, then a very precise correction for common Pb can be made using the measured 207Pb/206Pb ratio. This correction is iterative, for a calculation of f from
giving a corrected 206Pb/238U age, from which a revised f and age are derived. However, this method is evidently applicable only to zircon so young (Phanerozoic) that the discordance of individual analyses cannot be detected within the limits of analytical uncertainty. It produces a suite of radiogenic 206Pb/238U estimates that can be assessed for equivalence free of correlated errors propagated from the common Pb corrections. Any discordance and/or inheritance is apparently evident as excess scatter in the corrected 206Pb/238U values. This is not necessarily a valid reason for discarding data which doesn’t fit, because the errant values may cluster and the more reliable values may be scattered, so without objective independent standards for the true ages, such procedures are driven by the assumed evolutionary ages.
However, Schaltegger, Schmitt, and Horstwood (2015) stated that common Pb corrections are critical using SIMS because of the small volume of material consumed which renders Pb analyses vulnerable to surface contamination (especially affecting the accuracy of 207Pb/235U ages). The presence of non-radiogenic Pb (Pbc) also causes displacement of data points off the concordia, but their trajectory is controlled by the composition of 207Pbc/206Pbc. Proxies for common Pb (primarily 204Pb) typically show decreasing intensities with sputtering time, indicating that most non-radiogenic Pb is apparently surface derived. Thus, Schaltegger, Schmitt, and Horstwood (2015) reported that in the UCLA geochronology laboratory anthropogenic 207Pbc/206Pbc = 0.8283 is the default Pb composition for correcting zircon U-Pb analyses where common Pb intrinsic to the crystal lattice is believed to be extremely low. Like other geochronologists, they then indicated that the measures of common Pb used to determine corrections are 204Pb (stable), 207Pb (quasi-invariant for young zircon), or 208Pb (quasistable for zircon with high U/Th). Furthermore, the main causes for erroneous common Pb corrections are over- or under-counting of the monitor common Pb species, in particular for 204Pb. Under-counting can occur if peaks are mis-centered, which can typically be avoided by using nearby reference masses for peak centering (for example, 94Zr2O for 204Pb). Overcounts can result from interferences that are unresolved at the mass resolution M/ΔM = ~4500 at the 10% valley definition routinely used for SIMS U-Th-Pb geochronology. Practically unresolvable interferences, with their nominal mass resolution requirements in parenthesis, are 204Hg+ (500,000), 232Th144Nd16O22+ (50,000), or 186W18O+ (11,000). With the exception of 204Hg+ (sometimes due to contaminated Au targets used for applying the conductive coating), these interferences are much more detrimental for monazite (high Th) and rutile (high W) than for zircon. For monazite and rutile, peak-stripping from monitoring related species (for example, 202Hg+, 232Th144Nd16O22+, or 183W18O+, respectively), or the suppression of the interference using energy filtering, can mitigate their impact on the common Pb correction.
Determining the amount of common or initial Pb using LA-ICP-MS
In contrast, Košler and Sylvester (2003) maintained that using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) to date zircon does not usually require large common Pb corrections. They had found from experiments that most zircons from a wide range of rock types apparently contained so little common Pb that the correction was always insignificant. They suggested that most of the common Pb in a typical LA-ICP-MS analysis originates from the sample. Thus, its isotopic composition can be accurately estimated using models of Pb isotopic evolution (such as Stacey and Kramers 1975), or it can be derived from analysis of Pb isotopes in minerals cogenetic with the zircons (for example, feldspar). This and their other options—measuring the 204Pb, using the 208Pb, or the 207Pb method—are the same options suggested by Ireland and Williams (2003) already discussed for SIMS analyses.
Košler and Sylvester (2003) admitted that the major problem with the 204Pb method in LA-ICP-MS is the required precise measurement of the miniscule 204Pb. For zircons, which are claimed to contain very little common Pb, the 204Pb signal is overwhelmingly dominated by isobaric interference of 204Hg. Mercury is often present in trace amounts in various components of the LA-ICP-MS instrument, and it contributes to the 204 signal intensity. In theory, one could calculate and subtract the appropriate amount of 204Hg contributed to the 204 signal by measuring another Hg isotope and knowing the natural Hg isotopic composition. However, there is a large (several percent) uncertainty in the natural composition of Hg and thus the correction for highly radiogenic zircons could easily exceed 99%. Thus uncertainties associated with the common Pb correction would be too high to give a useful precision on the resulting radioisotope ages. In samples with a high common/radiogenic Pb ratio, Košler and Sylvester (2003) stated that the use of the 204 method is a viable option, especially if the 204Pb can be precisely and accurately determined using a multi-collector ICP-MS and the 204Hg signal can be suppressed. The necessity of the correction is then judged on whether the corrected 207Pb/206Pb lies outside the internal errors of the measured ratio.
Košler and Sylvester (2003) maintained that the 208 method is potentially useful for LA-ICP-MS analyses, as it does not suffer from isobaric interferences. It seems suitable for a wide range of Pb isotopic compositions, except for samples with a high Th/U ratio and thus high 208Pb. On the other hand, Košler and Sylvester (2003) claimed the 207 method is most appropriate for young samples that have a concordant U/Pb composition (that is, their respective U-Pb ages are the same). Due to their low 207Pb contents, the 207Pb/206Pb and 207Pb/235U ages of such samples would not yield a geologically useful precision (that is, in evolutionary time), and therefore their 206Pb/238U ages are usually preferred. However, Košler and Sylvester (2003) admitted that the assumption of concordancy in the 208 and 207 methods may not be valid for some young zircons which contain unsupported 206Pb and 207Pb, presumably due to incorporation of 230Th and 231Pa respectively (see figs. 1 and 2) during their crystallization (Schärer 1984; Schmitz and Bowring 2001) (which is an admission that contamination occurs).
Jackson et al. (2004) found that trying to measure the 204Pb in their samples in order to correct for the common Pb in them or that Pb added during the samples’ preparation for analyses proved fruitless owing to the overwhelming contribution to the signals from 204Hg. They also tested the common Pb correction procedure of Andersen (2002). However, that procedure is very sensitive to the measured 208Pb/232Th ratio and their samples were low in 208Pb and 232Th concentrations, so they could not use that method effectively. Instead they selectively integrated LA-ICP-MS time-resolved signals and graphical evaluation using the Tera-Wasserburg diagram.
Jackson et al. (2004) reasoned that in the LA-ICP-MS analysis the ablation surface penetrates into the sample on a scale where it may encounter significant chemical or isotopic variations related to alteration, inclusions, fractures, and inherited cores, and on a timescale they believed where transient signals related to these features are commonly resolvable if a fast data acquisition protocol is used. It should be noted, however, that the isotope distribution in the crystal cannot necessarily be considered uniform, so the surface sample cannot necessarily be considered representative. Thus they found that each analysis recorded a profile of the elemental and isotopic composition of the sample with depth. Because in many zircons it is believed that common Pb and Pb loss occurs in restricted domains (such as along fractures and rims), Jackson et al. (2004) argued they could recognize such domains easily in the time-resolved signals of ablations that penetrated into them. They used appropriate software to display the signals and their Pb isotopic ratios. They then selectively integrated only the most isotopically concordant portions of the signals, thereby hugely reducing the incidence of analyses they believed were affected by common Pb and Pb loss. However, it could be argued that this is not good science when such selections are made with an a priori assumption that the isotopically concordant portions of the signals must yield the “correct” age of the sample being analyzed because of believing they were less affected by essentially unknown and thus unquantified common Pb or Pb loss.
For younger zircons containing a significant amount of common Pb, Jackson et al. (2004) found that plotting their analytical data on Tera-Wasserburg diagrams was apparently the most effective method of evaluating, and correcting for, contributions from common Pb. Once plotted, only those data points which clustered together on the concordia were used to obtain a U-Pb date for the analyzed zircon grains. This is, of course, a selective choice of data which supports a pre-determined bias. Some data points plotted along an apparent discordia line, which was interpreted as due to common Pb. Data points intersecting the concordia that yielded older apparent U-Pb ages than the clustered concordant data points were interpreted to be due to inherited Pb. On the other hand, data points intersecting the concordia that yielded younger apparent U-Pb ages than the clustered concordant data points, and those having 207Pb/206Pb > 0.08 that plotted below the apparent discordia line, were interpreted to be due to radiogenic Pb loss and common Pb gain.
Chew, Petrus, and Kamber (2014) reviewed the U-Pb LA-ICP-MS dating of U-bearing accessory minerals such as apatite, titanite (sphene), and rutile with supposedly variable common Pb. They acknowledged that these minerals can accommodate a significant amount of initial Pb in their crystal structures and thus arguably the major limitation on the accuracy and precision of U-Pb dating of them is the need to use a common Pb correction. They noted that typically the common Pb corrections are undertaken on both age standards and unknowns using either concordia or isochron plots on a suite of co-genetic grains, or alternatively on individual analyses using an appropriate choice of initial Pb isotopic composition and employing several different methods (Ludwig 1998; Williams 1998).
Elaborating, Chew, Petrus, and Kamber (2014) pointed out that concordia or isochron plots on a suite of co-genetic grains (that is, grains of different minerals in the same rock unit) do not require an estimate of the initial Pb isotopic composition, but instead require a large spread in the radiogenic Pb/common Pb ratios. The total-U/Pb isochron, a three-dimensional 238U/206Pb versus 207Pb/206Pb versus 204Pb/206Pb plot (Ludwig 1998), does not assume concordance and apparently also yields the smallest error of any possible U-Pb or Pb-Pb isochron as all relevant isotope ratios are used at the same time. Other isochrons, such as the 238U/204Pb versus 206Pb/204Pb, 235U/204Pb versus 207Pb/204Pb, and 207Pb/204Pb versus 206Pb/204Pb plots, all assume the U/Pb* data (where Pb* is the radiogenic Pb component) are concordant to calculate accurate isochron dates. Another approach often employed in U-Pb dating of high common Pb phases involves projecting a straight line through the uncorrected data plotted on a Tera-Wasserburg concordia to determine the common Pb component on the 207Pb/206Pb axis. The 238U/206Pb age can then be calculated as either a lower intercept 238U/206Pb age on the concordia or as a weighted average of 207Pb-corrected ages using the concordia 207Pb/206Pb intercept as an estimate of the initial Pb isotopic composition. This approach also assumes that the U–Pb* data are concordant and equivalent.
Chew, Petrus, and Kamber (2014) also noted, as have others, that the alternative approach involves correcting individual analyses for initial Pb prior to age calculations. The best estimates of the initial Pb isotopic compositions are derived by analyzing a low-U co-genetic phase (for example, feldspar) which exhibits negligible ingrowth of radiogenic Pb. If such data are not available initial Pb can be estimated from Pb evolution models (for example, Stacey and Kramers 1975; Kramers and Tolstikhin 1997). Otherwise, three separate strategies exist, namely, the 204Pb-, 207Pb-, and 208Pb-correction methods (Williams 1998), as already discussed. The main advantage of the 204Pb correction method is that it does not assume U/Pb* concordance. It does require accurate measurement of 204Pb and is sensitive to the low 206Pb/204Pb ratios encountered in Phanerozoic samples, and is thus ideally suited to U-Pb dating by high-precision ID-TIMS or MC (multi-collector)-ICP-MS analyses. However, the ability to identify concordance in the 204Pb-corrected data can be obscured by an inappropriate choice of initial Pb. Both the 207Pb- and 208Pb-correction methods assume initial concordance in the 238U/206Pb-207Pb/206Pb and 238U/206Pb-208Pb/232Th space, respectively. The 207Pb-correction method is widely used in U-Pb ion microprobe (SIMS) studies, and only requires precisely measured 238U/206Pb and 207Pb/206Pb ratios and an appropriate choice of common Pb. The 208Pb-correction method is less commonly applied. It requires the measurement of 208Pb/206Pb and 232Th/238U, and an appropriate choice of initial 208Pb/206Pb, and works well for samples with low Th/U (<0.5) (Williams 1998). A variant on the 208Pb-correction method called the 208Pb (no Th) method is applied in U-Pb dating studies of rutile. The Th contents of granulite-facies rutile grains are often extremely low, meaning that all 208Pb measured can be attributed to common Pb, yielding a much more accurate common Pb correction than can be obtained using the 204Pb method (Zack et al. 2011).
Chew, Petrus, and Kamber (2014) provided details of the software they recommend laboratories use to compute common Pb corrections from LA-ICP-MS U-Th-Pb isotopic analyses via these various methods outlined already (Petrus and Kamber 2012). Yet they gave no details of whether that software had been checked independently for any computational errors. Nevertheless, they claimed it is a set of suitable procedures that work with the standard software package developed for processing and visualizing mass spectrometry data (Paton et al. 2010, 2011) to provide advanced U-Pb geochronology data reduction and visualization capabilities. It expands on the original U-Pb geochronology software package of Paton et al. (2010) by calculating 207Pb-206Pb ages and common Pb corrections for each time-slice of raw data, along with live concordia diagrams for visualizing of data while adjusting integration intervals. These live diagrams include conventional (Wetherill), inverse (Tera-Wasserburg), three-dimensional U-Th-Pb and total U-Pb concordias, and provide instantaneous feedback regarding discordance, uncertainty, error correlation, and common Pb. The original data reduction software package (Petrus and Kamber 2012) could undertake common Pb corrections on unknowns using an initial Pb isotope composition calculated using the Stacey and Kramers (1975) terrestrial Pb evolution model for the crystallization age of the accessory mineral in question. But both it and the U-Pb geochronology data reduction software package of Paton et al. (2010) assumed that the U-Pb reference standards are free of common Pb. Such an assumption is arbitrary, unsubstantiated, and not even reasonable, given that the U-Pb reference standards must be U-Pb radioisotope dated themselves. However, all the U-Pb apatite and titanite reference materials investigated contain appreciable (and usually variable) amounts of common Pb, and hence a common Pb correction needs to be applied to the reference materials prior to corrections for downhole fractionation and instrument drift. This data reduction scheme presented by Chew, Petrus and Kamber (2014) thus automatically undertakes common Pb corrections, using either the 204Pb, 207Pb or 208Pb (no Th) methods for determining the initial Pb isotope compositions, for the accompanying standard reference materials based on their known radiogenic and common Pb compositions (and “known ages”), so that those standards which contain variable common Pb can be employed as primary reference materials in U-Pb geochronology studies. Of course, the ages of these standard and primary reference materials must first be determined by U-Pb and other radioisotope dating methods, which involves the same unknown error factors and unverifiable assumptions in choosing their agreed ages, so these are not independent objective standards at all.
Similarly, Schaltegger, Schmitt, and Horstwood (2015) reported that one of the key problems for LA-ICP-MS U–Pb geochronology is the inability to precisely measure 204Pb for the correction of common Pb. Despite the time sacrifice for single collector (SC) and quadrupole (Q) ICP-MS systems in doing so, they advised that the two principal masses 202Hg and 204(Pb + Hg) should be monitored in any case, even imprecisely, to allow recognition of common Pb components clearly above background. Without this, the cause of discordant data points can only be surmised to be due to common Pb rather than demonstrated to be the case. Ultimately however, Schaltegger, Schmitt and Horstwood (2015) admitted that laser ablation is limited in its ability to correct for common Pb when compared to both ID-TIMS and SIMS. Equally, they warned that “blind acceptance” of common-Pb-corrected data, without scrutiny of the data scatter prior to correction, can mislead interpretations due to masking of non-analytical scatter by the increased data point uncertainties after correction. In addition, false impressions of concordance may result as data are forced to the concordia by assuming potentially inaccurate common Pb compositions for correction. They admitted that this is potentially a problem at all scales of precision for each of these instrument techniques for obtaining U-Pb isotopic analyses.
Thus, Schaltegger, Schmitt, and Horstwood (2015) suggested that a better way of assessing common-Pb-affected data is to first view it without the common Pb correction on a Tera-Wasserburg (207Pb/206Pb versus 238U/206Pb) diagram so that the true scatter in the data can be seen, as well as the trend in the data indicating the appropriate composition to use for correction. Sometimes this trend is not visible within a cluster of data, and the analyst has no option but to assume a relevant composition based on other information. However, particularly for non-zircon accessory phases, common-Pb compositional constraints may be determined from U-Pb data with excess scatter, by initial plotting of data that are not corrected for common Pb, prior to the expansion of the uncertainties due to the correction. Thus this procedure allows the smaller data point uncertainties to better resolve these components. Therefore, Schaltegger, Schmitt, and Horstwood (2015) suggested that better resolution of the true scatter is important in defining whether the data do indeed represent a single population, the fundamental assumption which must be adhered to if the data are to be corrected. A common-Pb-corrected age and uncertainty can then be more appropriately defined in this way. Equivalent to a 207Pb-based common Pb correction, this approach still ignores, however, the potential for Pb-loss to also disturb the system, except in that Pb loss in addition to common Pb would likely result in increased scatter of the data population now better resolved with the uncorrected, more precise, data point uncertainties. Schaltegger, Schmitt, and Horstwood (2015) noted that Andersen (2002) had highlighted the limitations of 207Pb and 208Pb-based common Pb corrections in the presence of Pb loss and illustrated an alternative approach, not requiring measurement of 204Pb (particularly for ICPMS- based measurements), which accounted for nonzero- age Pb loss within a data population. The potential for systematic errors after correction were however noted at Pb-loss proportions greater than a few percent.
Horstwood et al. (2016) presented the workflow for the recommended uncertainty propagation protocol for LA-ICP-MS U-(Th-)Pb data, including how to correct for common Pb. At one stage in this workflow when the gas blank is measured, they highlighted the need to calculate the uncertainty if an average of the gas blank is being applied to the whole analysis. Then the ablation signal intensities need to be measured and thus calculate the blank subtracted signal intensities. If correcting for common Pb on each signal intensity measurement, this correction should then be made.
Horstwood et al. (2016) asserted that next in the workflow if a common Pb correction is made based on the average common Pb measurement for the whole of the ablation/selection, then the correction should be made next and the appropriate uncertainty propagated. If a common Pb correction has been made using compositions based on independently measured values (for example, the Pb isotope composition of feldspar) or a composition likely to be variable within the host material, then this common Pb compositional variability/uncertainty should be propagated accordingly. If a model Pb composition is being used based on Pb isotope evolution through time (for example, Stacey and Kramers 1975), this propagation is made later.
Having obtained the igneous or metamorphic population uncertainty, Horstwood et al. (2016) stipulated that next the systematic uncertainties should now be propagated to quote the result as an age. These uncertainties are:
- the ratio uncertainty of the primary reference material used for normalization (noting that the age of the primary reference material must first be determined by U-Pb and other radioisotope dating methods, which involves the same unknown error factors and unverifiable assumptions in choosing its agreed age, so this is not an independent objective standard at all),
- the long-term excess variance of the validation materials,
- the decay constant uncertainties, and
- the model Pb ratio uncertainty (for example, using the Pb-evolution model of Stacey and Kramers 1975) used for the common Pb correction if based on the measurement average.
These uncertainties are then propagated, since systematic uncertainties constitute limiting uncertainties (the uncertainty level below which the final uncertainty cannot be quoted) and cannot be reduced by increasing the number of data points. By including these uncertainties earlier in the propagation, they would evidently be reduced erroneously during the definition of the population uncertainty. Horstwood et al. (2016) noted that the uncertainty related to systematic errors must be applied to the ratio uncertainty before calculation of the age uncertainty. It is not clear whether they advocate the propagation of the errors in the actual age calculation, but it would appear they imply it.
When processing LA-ICP-MS U-Pb data, a primary reference material is used to calibrate or “correct” the data for the sample and validation materials. To obtain accurate results, Horstwood et al. (2016) maintained it is vital that reference values for the primary material are well characterized (which would generally be determined by isotope dilution methods), and that these values appropriately describe the actual material ablated. For example, zircon can lose radiogenic Pb, and monazite can incorporate variable amounts of common Pb and/ or build up excess 206Pb due to the incorporation of extra 230Th at the time of crystallization. For these reasons, Horstwood et al. (2016) stated it is not valid to ask the question “What is the age of the reference material?” That will vary depending on whether one is describing the 207Pb-206Pb, 206Pb-238U, 207Pb-235U, or 208Pb-232Th age, or the concordia age which represents a statistical weighting of two of these (Ludwig 1998). Instead, they maintained that it is the relevant isotope ratio that must be used to normalize the corresponding data for the sample and validation materials. A reference ratio including or excluding common Pb and/or 230Th corrections should be used depending on whether this correction has been made to the LA-ICP-MS data at this point.
Although ID-TIMS data are the benchmark reference data for LA-ICP-MS geochronology, Horstwood et al. (2016) noted that these data are typically tabulated as ratios corrected for common Pb, with dates additionally corrected for 230Th disequilibrium. Unless these corrections have already been made to the reference LA-ICP-MS data (and this is generally not the case), these ages and ratios will be the wrong reference values to use for calibrating LA-ICP-MS analyses. In other words, the LA-ICP-MS U-Pb ages are only as good as the quality of the common-Pb-corrected data for the reference materials.
For example, using the data of Wiedenbeck et al. (1995), Horstwood et al. (2016) found that zircon standard 91500 consistently demonstrates slight discordance, resulting in a lower 206Pb-238U ratio (and age of 1062.4 Ma) than would be expected if assumed concordant at the age given by its 207Pb- 206Pb ratio (1065.4 Ma). This represents a potential 0.3% inaccuracy in one of the normalization factors if only one of these ages is assumed to represent the correct “age” of the material. However, Horstwood et al. (2016) demonstrated an even larger inaccuracy effect for zircon standard GJ1. The difference in its respective ages represented by the 206Pb-238U and 207Pb-206Pb ratios is 1%. Their new ID-TIMS U-Pb data for GJ1 obtained using chemical abrasion (Mattinson 2005), the EARTHTIME tracer (Condon et al. 2015; McLean et al. 2015), and the 238U/235U ratio defined in Hiess et al. (2012), replicated the results for GJ1 of Jackson et al. (2004) (without chemical abrasion), but with significantly greater precision, demonstrating that standard is measurably discordant. Thus, Horstwood et al. (2016) insisted that consistent apparent discordance must be reflected in the reference values used for normalizing LA-ICP-MS data. And the cause of this discordance at least in part stems from inaccuracy in the decay constant of 235U relative to the decay constant of 238U (Mattinson 2010; Schoene et al. 2006), and the variability in the 238U/235U ratio (Snelling 2017).
Horstwood et al. (2016) also demonstrated that the same features can be seen in monazite reference material data where common Pb and excess 206Pb from initial 230Th disequilibrium result in an otherwise reversely discordant “Stern” monazite standard being “concordant” at an age older than its true age (approximately 512 Ma versus 507 Ma). This “concordant” material is, however, the composition of material sampled during laser ablation analysis and therefore provides the correct reference ratios that should be used to define normalization factors if common Pb and Th corrections are not to be applied to the data prior to normalization.
Therefore, the detailed examination of zircon and monazite reference materials by Horstwood et al. (2016) demonstrated that a single “reference age” should not be used to derive expected U/Pb, Th/Pb, and Pb/Pb ratios for a material. Instead, the ID-TIMS determined ratio uncorrected for initial common Pb and 230Th disequilibrium should be used (assuming that these corrections have not already been made to the laser ablation data). The example of zircon standard GJ1 also demonstrated that if a single reference age is taken as the concordia age of the ID-TIMS data set, both Pb/Pb and U/Pb reference values will be inaccurate, because the concordia age is not equivalent to either ratio value. Thus, Horstwood et al. (2016) concluded that since a number of non-zircon U-containing minerals are required as reference materials but show variable common Pb contents (for example, Chew, Petrus, and Kamber 2014), a preferable way of defining the reference material compositions is through “reference models” which have this variability built into the calculations, as per McLean, Bowring, and Gehrels (2016).
Horstwood et al. (2016) recommended minimum analytical data to be submitted for publication. That would record details of the sample, ablation signal, concentration, proportion of common Pb, and Pb-Pb, U-Pb and Th-Pb (if measured) isotope ratios, with and without common Pb correction if appropriate, the date and concordance of the data points. The size of at least one ion beam signal should be included to allow all the others to be estimated via the ratios reported. The measured ion beam sizes and knowledge from the metadata table of the detectors used for the different ion beams would allow others to assess the reported precision levels and the likely analytical limitations within the data. All these necessary reporting details illustrate how difficult it is to accurately determine what is the true age of the rock sample under investigation. If there is so much difficulty accurately analyzing the reference materials, then how dependable is the resultant sample age?
Horstwood et al. (2016) also insisted that the proportion of common Pb within the analysis should be expressed as the proportion of the 206Pb ion beam that is non-radiogenic Pb, calculated as:
where 206Pb204Pbmodel is the 206Pb/204Pb ratio (for example, using the Pb-evolution model of Stacey and Kramers 1975) at the apparent (non-common Pb corrected) 207Pb-206Pb age. Expressed in this way, LA-ICP-MS U-(Th-)Pb data can then be compared readily to the established reporting protocol of the SIMS community, which represents a direct analog of the data produced by LA-ICP-MS. Reporting of ratios in a configuration ready for plotting as a Tera and Wasserburg (1972) concordia plot (without common Pb correction) as well as a Wetherill (1956) concordia plot (with common Pb correction if made) with a correlation coefficient (rho) allows others to more easily replicate the plots described instead of requiring them to recalculate ratios and rho values which could lead to errors in rearranging data. The uncertainty level on the concentration estimates should also be described, highlighting which data are or are not corrected for common Pb, how the concordance values have been calculated, the systematic uncertainty components and their values, and any relevant references, for example, the source of the decay constants, the 238U/235U ratio, and especially the source of the primary reference values used for normalization.
Similarly, McLean, Bowring, and Gehrels (2016) contended that the LA-ICP-MS community is now faced with archiving a rapidly-growing quantity of U-Pb geochronology data with associated analytical results. Most importantly, the challenge being faced is to ensure that the data meet the highest standards for precision and accuracy, and that interlaboratory biases are minimized. However, there is little consensus with regard to analytical strategies and data reduction protocols for LA-ICP-MS geochronology. The result is systematic interlaboratory bias, and both underestimation and overestimation of uncertainties on calculated dates. This situation only decreases the value of data in repositories where the data is archived with the analytical results from participating laboratories.
Thus McLean, Bowring, and Gehrels (2016) presented free open-source software that implements new algorithms for evaluating and resolving many of these discrepancies. Their solution is the result of a collaborative effort to extend the U-Pb_Redux software used by the ID-TIMS community to the LA-ICP-MS community. Named ET_Redux, their new software automates the analytical and scientific workflows of data acquisition, statistical filtering, data analysis and interpretation, publication, community-based archiving, and the compilation and comparison of data from different laboratories to support collaborative science. Of course, one of the main problems with automated software is that if users do not understand and appreciate what the software does and what the quality of the input data should be, then the resultant recorded output will not be all it is supposed to be, and thus as good as others are expecting it to be!
According to McLean, Bowring, and Gehrels (2016), ET_Redux propagates uncertainties from the measured intensities of ion beams through to radioisotopic U-Th-Pb dates (corrected for initial common Pb where appropriate), and quantitative interpretations based on those dates, such as weighted means, regressions through discordant arrays of data, and kernel density estimates. They pointed out that all data reduction protocols generally follow the same established pattern. That starts with a background or gas blank subtraction from an on-peak measurement, then correction for any isobaric interferences (for example, 204Hg on 204Pb). This is followed by calculation of the relative abundances of U, Th, and Pb isotopes throughout the ablation, utilizing a set of bracketing reference materials to quantify laser and mass-spectrometer-induced elemental and isotopic fractionation, applying these average or time-dependent corrections to simultaneously measured unknowns, performing a common Pb correction if necessary, and then often interpreting multiple unknown analyses together, for instance in a weighted mean or kernel density function. Data reduction and uncertainty propagation in ET_Redux follows this blueprint. However, it utilizes the innovative mathematical approaches described by McLean, Bowring, and Gehrels (2016), employing the matrix-based full uncertainty propagation protocols described in McLean, Bowring, and Bowring (2011).
As described by McLean, Bowring, and Gehrels (2016), this ET_Redux software supports two different common Pb (Pbc) correction protocols. For samples where 204Pb is both measured and above the detection limit, defined as 204Pb–2σ ≥ 0, a 204Pb-based correction is made based on either a user-input Pbc isotopic composition or a Pb isotopic composition whose Stacey-Kramers common-Pb age (Stacey and Kramers 1975) agrees with the 206Pb-207Pb, 206Pb-238U, or 208Pb-232Th date, as chosen by the user. For the Stacey-Kramers option, this agreement is reached quickly via an iterative problem solving routine. If there is no usable 204Pb data, then a 207Pb-based correction can be made using a user-input or Stacey- Kramers model 206Pb/207Pb ratio. This makes the assumption that the unknown analyses are perfectly concordant, so ET_Redux will not plot them on a concordia diagram and will only give the resulting corrected dates in the data table.
McLean, Bowring, and Gehrels (2016) indicated that there are two different types of uncertainty in the Pbc isotopic composition required when evaluating the weighted mean of multiple analyses. The first is an estimate of the grain-to-grain variability in the Pbc isotopic composition. That is propagated as a random contribution to a weighted mean uncertainty and can be reduced as the number n of analyses included in the weighted mean increases. The second estimated uncertainty is the systematic uncertainty in the mean of the Pbc isotopic composition, which is propagated into weighted means and does not decrease with n. Clearly, if there is grain-to-grain variability in the common Pb isotopic composition, then it only increases the uncertainty of whether the common Pb has been correctly identified and accurately measured, and thus increases the uncertainty of whether the resultant calculated age for the sample is really its true age.
Initial or common Pb still unresolvable
That common or initial Pb is still a major problem for U-Pb dating, even after the establishment of these protocols and statistical “manipulations,” is demonstrated by the most recent work by Tera (2017a, b). Indeed, Tera (2017a) noted that implicit in the term “initial Pb” is the existence of an isotopically homogeneous source (or reservoir) with a specific Pb isotopic composition, from which the rocks of a given terrain were originally extracted at some time supposedly millions or billions of years ago as a magma that acquired the source’s isotopic composition at that time. Evidence generally substantiating that simple scenario was presented by Tera (2006), who showed that accurate Pb-Pb isochrons of surface rocks on the common Pb plot fall into subgroups where the isochrons of each subgroup intersect each other at a single point apparently corresponding to the present-day composition of a parental reservoir, from which the rocks of a subgroup were extracted. He argued that if this is valid, then the earth has had many magma reservoirs, formed at different times, each having a distinct isotopic composition.
According to Tera (2017a), the weakness of this method is the lack of independent evidence relating an isochron to an inferred reservoir. The slope of the line is said to equal the in situ decay ratio *207Pb/206Pb, which yields the age. In contrast, on the 204Pb/206Pb versus 207Pb/206Pb plot the intercept of an isochron with the y-axis yields the *207Pb/206Pb ratio, while the initial Pb isotopic composition (if it is determined) would fall on the other end of the isochron, which is then easily equated with a mixing line with potentially two visible end-members.
Tera (2017a) reasoned that subsequent to emplacement of a terrain, its rocks (specifically, the minerals within them) may have had their elements, including U, Th, and Pb, mobilized and remobilized through bombardment, heat, and other metamorphic agents, in events referred to collectively as “disturbances.” These disturbances are generally not identified by geochronologists, and are inferred from their effect on the isotopic make-up of the samples. These disturbances may be strong enough to change the isotopic composition of some or all the rocks of a terrain, but they are likely too impotent to reset the isotopic composition of initial Pb. Such resetting would probably require melting the entire terrain or at least a very large portion of it, which sometimes does happen if the intensity of metamorphism results in partial melting to produce a granitic magma. Otherwise, irrespective of the multitude of superimposed disturbances on a terrain, he argued that the minerals and their rocks (which rarely, perhaps never, lose all their Pb) will continue to carry within them the very same initial Pb isotopic composition they all inherited from their source.
Tera (2017a) thus proposed a method for determining the initial or common Pb of a terrain on the basis of the measured Pb isotopic compositions of its rocks. The method was inspired by the premise stated above that the initial Pb inherited by the rocks from a reservoir from which they were extracted is immutable and inerasable, irrespective of the multitude of disturbances that may have subsequently been superimposed on the terrain. Of course, this in itself is a debatable assumption. He argued that if this rationale is valid, then a large Pb isotope database (including data on mineral separates with low affinities for U and Th) that is representative of a terrain, when plotted on any Pb isotope correlation diagram (for example, the conventional Pb-Pb plot), may define a dispersion field that tapers toward a single spot. That single spot (once unambiguously determined) would be the initial Pb isotopic composition for that terrain (as interpreted).
Furthermore, from the equations of radioactive decay as applied in geochronology, Tera (2017a) claimed that there was evidence for the potential existence within the Pb isotope dispersion field, for the rocks within any particular terrain, of four classes of lines that converge in different types of Pb isotope correlations to always meet in a point that yields the composition of the initial Pb for that terrain. These Pb isotope correlation lines as he defined them are:
- isochrons—defined by samples that remained as closed systems since crystallization;
- transposichrons—each made up of samples that experienced fractionation in a disturbance episode by the same constant factor F = (238U/204Pb)pc/(238U/204Pb)cr, and/or the same constant factor K = (232Th/238U)pc/(232Th/238U)cr, where the subscript pc signifies a value resulting from superimposed post-crystallization event(s), and the subscript cr indicates a value that remained unaltered since the rocks’ extraction (that is, since the minerals crystallized);
- heterochrons—each defined by samples produced by different Pb isotopic evolution scenarios (including different multiple stages with different values for the geochemical parameters F and K), which happen to accidentally have the same average F and/or the same average K; and
- mixichrons—defined as real or hypothetical mixing lines with possibly determinable endmembers, one being the actual composition of the initial Pb of the samples, and the other being the isotopic ratio of the in situ decay Pb.
Yet there is no definitive, objective way to determine the difference between an isochron and a mixichron (mixing line).
Tera (2017a) demonstrated by synthetic examples that heterochrons seem to occur because the production of a Pb isotopic ratio by radioactive decay is controlled by multiple independent variables. He claimed that this circumstance allows for various combinations of the parameters to “accidentally” produce the same radiogenic isotopic ratio. This of course begs the question as to how it is determined which are the “accidentally” produced isotopic ratios, and which are the real radiogenic isotopic ratios. Nevertheless, Tera (2017a) presented an application to a terrestrial terrain, one of the four he separately discussed in a companion paper (Tera 2017b), which he claimed further illustrated the validity of the rationale and the practicality of his proposed method. Tera (2017a) integrated the Pb isotopic ratios from the rocks in a terrain into his methodology through what he called “Thorogenic, Uranogenic, Lead Isotope Plains” or TULIPs. These are correlation diagrams combining the three isotopes 208Pb, 207Pb, and 206Pb. They may include in addition the nonradiogenic isotope 204Pb. He found that on certain TULIPs some of the disturbed Pb isotopic data of a terrain fell in subgroups each defining a line having a calculated specific fractionation factor K = 232Th/238U. The majority of such lines may converge to meet in a single point, which yields the initial Pb isotope composition of that terrain (that is, by his interpretation). He went on to present theoretical considerations, numerical demonstrations, and an application to a terrestrial terrain.
On the subject of how the crystallization ages of rocks from various terrains are determined, Tera (2017a) admitted this was thought to have already been established by Tera (2006). However, in view of now finding the possibility of widespread heterochrons, he concluded that the determination of accurate crystallization ages by the Pb-Pb method alone may remain subject to some ambiguity. Nevertheless, he described a methodology for the determination of the isotopic composition of initial Pb that in his assessment is both explicit and accurate, acquiring this initial Pb parameter that seems to meet the conditions for crystallization ages of rocks in the same terrain.
Of course, as a committed uniformitarian naturalist, Tera (2017a) maintained that the determination of the initial Pb composition of each terrain in this fashion is a necessary step toward elucidation of the early evolutionary history of the earth, that is, its condensation from colliding matter from the solar nebula. But on its own, he admitted, such a step falls short of reaching that goal. Interestingly, he also admitted that an accepted precise age for the earth is not yet available, although “arbitrarily” he adopted an age of 4.563 Ga. Nevertheless, he conceded that full chronometric elucidation of earth’s history requires complete resolution of the isotopic composition of initial Pb into all the presumed multiple stages that led to its final composition. As he then admitted, this is not readily tenable.
So Tera (2017a) proposed a procedure introduced as an alternative that he termed “Congruently Associated Profiles” or CAPs, in which the initial Pb isotopic composition is partially resolved into two or three stages. According to Tera (2017a), in general the initial Pb isotopic composition of a terrestrial terrain evolved in more than one stage. He maintained that a method for determination of all the stages of initial Pb isotopic evolution is necessary for complete outlining of the earth’s history. But he admitted such a method does not exist, and may not even be possible to produce. However, he still claimed resolution to two and three stages (depending on certain constraints) is possible, with the dates of these stages being “congruent approximations.” So according to Tera (2017a) resolving the initial Pb isotopic composition to three stages becomes possible, if at least two terrains were extracted from the same reservoir. But if a reservoir has only one known terrain extracted from it, then it is not possible to resolve the initial Pb isotope composition to more than two stages. In addition, both the age of a reservoir and its isotopic composition are determinable only if more than one terrain were extracted from it. Nevertheless, Tera (2017a) remained hopeful that now the initial Pb isotopic composition has been shown to be routinely determinable according to his method, the resolution of its multi-stages may someday become possible.
In his companion paper, Tera (2017b) applied his TULIP methodology for determining the initial Pb isotopic composition based on the measured Pb isotopic compositions of many rocks in a terrain to four terrestrial terrains. He gave particular emphasis to the determination of the initial Pb isotope composition of the so-called South of Isua terrain in Greenland, because of the availability of a large high-quality Pb isotope database on its rocks and feldspar separates. Accordingly, his results for this terrain demonstrated the apparent feasibility of routine determination of the initial Pb isotopic composition by his developed methodology, once large databases for such terrains are established. Yet even in terms of the caveats admitted by Tera (2017a, b), if there is a lack of independent evidence relating an isochron to an inferred parent reservoir from which the rocks of a terrain were extracted, then there can be no certainty that the initial Pb isotopic composition determined for a terrain by his methodology is in fact the true value for the initial Pb isotopic composition.
Tera (2017b) explained that the projection of the initial Pb isotopic composition by using the database of Pb isotopic ratios for a terrain’s rocks is caused by Th-U-Pb fractionation, in disturbing events superimposed on the rocks of a terrain. As a consequence, elemental fractionation can induce alignments of the Pb isotope data in linear trends of false “ages,” which together with the rocks’ isochron converge (on various Pb-Pb isotope plots) on a point that seems to yield the initial Pb isotope composition. He also found that this may result in two counter effects:
- the precise determination of the initial Pb isotope composition, and
- ambiguity in the exact meaning of an isochron (because of the possibility it was affected by fractionation, like the other lines).
Consequently, associating a Pb-Pb age with the determined initial Pb isotope composition may not always be meaningful.
Tera (2017b) again claimed that the ability to determine the initial Pb isotope composition opens the possibility for eventual unfolding of details of the assumed evolutionary history of the earth. However, he again admitted that cannot be satisfactorily achieved without additionally developing a methodology for resolving the initial Pb isotopic composition into its multi-stages. Because such a methodology was lacking, he developed the procedure of Congruently Associated Profiles (CAPs) for resolving the initial Pb isotope composition to a maximum of three stages, as outlined in Tera (2017a). For his application of this methodology to the South of Isua terrain in Greenland, only a two-stage CAP solution was possible. It indicated to him a U-Pb fractionation event at 67.5 Ma after the earth’s accretion from the solar nebula (that is, a first stage of Pb isotopic evolution lasting from 4.563 to 4.496 Ga), with 238U/204Pb = 0.45 ± 0.25. For the second stage, extending from 4.496 to 3.8 Ga, he obtained 238U/204Pb = 9.25 ± 0.02. He concluded that the stated duration of 67.5 Ma for the first stage had to only be an upper limit, and in a multi-stage solution, if and when it became possible, the first stage duration would be shorter, and its meaning may be elucidated. As he admitted, for now “it remains subject to qualitative speculations” including the possibility of being associated with core formation, or an early impact (resulting in formation of the moon?). This ambiguity in his evolutionary speculations underlined to him the crucial need for a methodology to resolve the initial Pb isotopic composition to more of its multistages. But such “qualitative speculations” are hardly the basis for dogmatic assertions about a precise age for the earth, or accurate absolute U-Pb radioisotope ages for the earth’s rocks.
Discussion
Before proceeding, it is important to clarify some definitions of crucial terms, because there is even confusion over them in the geochronology community. Common Pb and initial Pb are terms sometimes used synonymously, but they are not necessarily the same. Common Pb can be defined as the isotopic composition of the Pb in the rocks in a region that had a common origin in a mantle or crustal reservoir from which they were extracted. On the other hand, initial Pb would be the isotopic composition of the Pb that initially was in a mineral or rock when it formed, that is, the Pb it inherited. Often the common and initial Pb in a mineral or rock may be the same. However, sometimes the formation of a mineral or rock may involve fractionation, extraction, and/or partitioning processes that may not transfer all the common Pb in the source to the mineral or rock when it forms, so that the isotopic composition of the inherited initial Pb may be different to that of the common Pb in the source from which it was formed.
By now it should be very clear that in spite of claims to the contrary the problem of knowing the initial Pb isotopic composition in a mineral or rock is still a problem to geochronologists trying to use the U-Pb radioisotope method to date rocks and minerals. For several decades they have followed a similar routine of assigning all the measured 204Pb in samples to the common or initial Pb component of the samples’ overall Pb isotopic compositions. And they have generally assumed that the common Pb and the initial Pb are essentially the same. Several procedures have been adopted according to the instrument and analytical technique being used (IDTIMS, SIMS, or LA-ICP-MS) to measure the 204Pb isotopic composition of the samples being dated and to thus determine what component of that is common or initial Pb.
The first option is to measure the amount of 204Pb directly. But the problem with that approach is the interference from the 204Hg signal. So the second option is to measure the Pb isotopic composition of a cogenetic mineral in the same or a related rock or ore of the same assumed age that contains no (or negligible) U (and Th) (and thus no radiogenic Pb isotopes), and then assign that Pb isotopic composition to be the value for the common or initial Pb in the rock or mineral being dated. This is supposed to be viable for “old” Precambrian rocks. For younger Phanerozoic rocks and minerals, the claimed most effective third method of evaluating and correcting for contributions from common or initial Pb has been to plot the analytical data on Tera-Wasserburg diagrams where only those points that plot near the concordia are then used to determine the ages, and any discordia is due to the common or initial Pb. Then the fourth method is to estimate the initial or common Pb isotopic composition in a sample by using the Stacey and Kramers (1975) Pb evolution model based on the sample’s presumed age. And the fifth method uses the 208Pb/206Pb ratio to estimate the initial or common Pb composition, but it only works for minerals with low Th/U ratios. Finally, if a mineral’s U-Pb ages are concordant (in agreement), then the sixth method uses the measured 207Pb/206Pb ratio to obtain what is claimed to be a very precise correction for common or initial Pb.
However, one other major issue facing geochronologists in their quest to determine the common or initial Pb in the samples they analyze is the problem of sources of contamination within their laboratories. Such sources include airborne particulates, labware, reagents, and the procedural blanks used in the analyses. And where polished surfaces of minerals are analyzed by an ion beam or laser ablation, there can be Pb contamination from the polishing compounds and any coating materials. To combat this, most laboratories have tried to characterize the Pb isotopic compositions of all these sources. Results often correspond to their geographic locations and Pb isotopic compositions of their local sources of Pb ores. Yet the contributions from all these sources are known to potentially change over time, so most laboratories have adopted Pb isotopic compositions for their procedural blanks with realistically large uncertainties that include temporal variabilities.
Measuring ratios, not absolute quantities
However, if these problems are not enough to deal with, there is one other major pitfall that must be overcome. Many different instrumental configurations for obtaining the material from a rock or mineral to be analyzed are used with mass spectrometers in U-Pb dating. Yet mass spectrometers are designed and operated to primarily measure isotopic ratios, not the absolute quantities of the individual isotopes. This is rarely mentioned or discussed in the conventional literature. Yet McLean, Bowring, and Gehrels (2016, 2482) have admitted: “We are interested in the relative abundances of isotopes present, usually expressed as ratios, and rarely require or have information on their absolute abundance to the same precision.” In other words, the absolute quantity of 204Pb in samples cannot be measured with certainty. And any attempt to directly measure the absolute quantity of 204Pb with sufficient accuracy is stymied by interference from the 204Hg signal.
This is not a trivial matter, because it is assumed that all the measured 204Pb, the only stable Pb isotope not derived by radioactive decay from a precursor radioisotope, is the most significant component of the common or initial Pb isotopic composition. Yet measuring the absolute amounts of 204Pb in samples is the only way those amounts can be known without recourse to assumptions. Every one of the other methods to determine the common or initial Pb isotopic composition mentioned above involves using the measured Pb isotopic ratios and assumptions. Ratios are simply that. The only way to determine an absolute amount of 204Pb from them is to make assumptions about the past history of the Pb isotopes in the samples, especially a deep time history for the earth and its origin, as well as for a deep time history for the samples being dated (for example, the Pb-evolution models). Yet the U-Pb radioisotope ages derived using those assumptions are then used to construct that deep time history. So, the outcome is model dependent, and the model chosen will be dependent on one’s worldview.
It should also be highlighted here that the analytical procedure to obtain U-Pb dates for samples of unknown ages usually requires the concurrent U-Pb analyses of standards or reference materials of supposed known ages. This is done to help quantify the common or initial Pb in the samples of unknown ages so that U-Pb ages can be derived for them. However, this merely shifts the analytical burdens of proof to the standards of supposed known ages. ID-TIMS analyses are often used on those standards to determine the isotopic composition of their common or initial Pb and thus their U-Pb ages. But as we have seen, even the ID-TIMS methodology requires the use of all the various procedures to determine the common or initial Pb isotopic compositions as already outlined, including their attendant problems and the assumptions made in those various procedures. The so-called 206Pb, 207Pb, and 208Pb methods for determining the common or initial Pb all require assumptions about the mineral (zircon) or rock’s history and depend on interpreting the measured Pb isotopic ratios of the mineral or rock and their U-Th-Pb ages within an assumed deep time history.
Primordial Pb
Furthermore, what is always never mentioned is the foundational assumption upon which the chain of assumptions is built, namely, that there was a particular primordial Pb isotopic composition. It is specifically assumed that all the solar system’s components were formed out of the solar nebula, including the earth and the asteroids (from which most meteorites are derived), and they had a particular initial Pb isotopic composition. This is the primordial Pb, not to be confused with the common or initial Pb in minerals and rocks which formed subsequently to the earth’s primordial formation in the uniformitarians’ assumed deep time history of the earth.
Details of how that primordial Pb composition was determined have already been provided above. It was Patterson (1956) who first concluded that the age of the earth is essentially the same as that of the meteorites (see fig. 6 again), and that the isotopic composition of the earth’s primordial Pb could be closely approximated by the Pb in meteoritic troilite. This was based on the assumption that the meteorites are regarded as fragments of larger bodies (mostly asteroids) that formed from the solar nebula along with the earth early in the history of the solar system. During the formation of the meteorites’ parent bodies it is conjected that the iron sulfide mineral troilite (FeS) formed. It is virtually free of U and Th, so the appreciable concentration of Pb in it has then been deemed the initial common Pb, or simply the primordial (first or original) Pb. Therefore, the isotopic composition of this Pb in this troilite is believed to have remained very nearly constant since crystallization. It appears to be the least radiogenic Pb available, that is, Pb containing the lowest quantities of 206Pb, 207Pb, and 208Pb. As such it is believed to be the closest representative of the isotopic composition of the earth’s primordial Pb, because the earth and the meteorites’ parent bodies are believed to have formed at the same time from an isotopically homogeneous solar nebula. This chain of reasoning involves rather speculative assumptions based on models which are in serious doubt.
The values for the isotopic ratios of this primordial Pb were first determined by Tatsumoto, Knight, and Allègre (1973) who analyzed the Pb in the troilite from the Canyon Diablo iron meteorite. They were later confirmed by Chen and Wasserburg (1983) (see table 3 again). Tera (1983) maintained emphatically that any allegations contrary to the earth’s primordial Pb being that in the Canyon Diablo troilite are ill founded, which is a rather subjective approach to a scientific proposition when he did not provide any objective evidence to support this emphatic claim. Notice that this primordial Pb isotopic composition is expressed in terms of Pb isotopic ratios and not in absolute amounts of the four stable Pb isotopes, including 204Pb. And notice especially that this implies that the earth thus had an initial endowment of 206Pb, 207Pb, and 208Pb, which therefore was not derived from radioactive decay of 238U, 235U, and 232Th respectively.
Of course, since some U and Th isotopes were apparently in the troilite when it formed, some of the radiogenic Pb isotopes have been derived since then by the decay of those U and Th isotopes, which have thus simultaneously been lost due to that decay. This only serves to add to the dilemma of just how much of each of the four stable Pb isotopes were really in the primordial troilite Pb. Uniformitarians simply calculate from the present measured U, Th, and Pb isotopic composition of the troilite back through 4.56 billion years to the supposed time when the troilite formed. But that again assumes a deep time history to provide/prove a 4.56 Ga age and history for the troilite, which is circular reasoning.
This then has consequences for the compositional makeup of the Pb in all minerals and rocks subsequently formed during the earth’s history, through the evolutionists’ assumed deep time history. This is because once U and Th radioactive decay starts in the first-formed minerals and rocks, the isotopic composition of their contained Pb starts changing, as more 206Pb, 207Pb, and 208Pb gets added to the primordial Pb. And then some or all of that Pb (and/or U and/or Th) is inherited by later rocks and their minerals formed from that primordial material and its primordial Pb (and U and Th). This of course is the basis for the uniformitarians’ Pb isotopic evolution models.
So this all means that the isotopic composition of Pb in a primordial rock or mineral subsequent to the earth’s initial formation consists of that primordial Pb, plus the Pb isotopes which formed subsequently in the primordial mineral or rock due to radioactive decay of U and Th. However, most (if not all) minerals and rocks are not primordial, but have formed subsequent to the earth’s primordial formation. Various events subsequent to the earth’s formation have caused new minerals and rocks to be formed. Each time that has happened during the earth’s history some or all of the U, Th, and/or Pb isotopes in the sources from which the minerals and rocks formed was incorporated into the new minerals and rocks based on the conditions of formation, the abilities of the minerals to incorporate U, Th, and Pb isotopes into their crystalline structures, and other factors. Yet it is normally assumed the radioisotope clocks have been reset when the new minerals and rocks form, except for the presence of the initial or common Pb. Furthermore, subsequent events may again form new minerals and rocks, which again changed the Pb isotopic compositions of the resultant minerals and rocks. Thus, the minerals and rocks we sample today may have resulted from repeated events which recycled more or less U, Th, and Pb isotopes multiple times in various ways, making it extremely difficult to interpret the history and meaning of the Pb isotope ratios measured today. That this is so is well illustrated in the case studies used by Tera (2003) to attempt to unravel the meanings and implications of today’s measured Pb isotope ratios in those minerals and rocks.
Pb isotopic evolution models
This brings us to consider the uniformitarian Pb isotopic evolution models in use. Whereas the first model proposed was that of Holmes (1946) and Houtermans (1946) (see fig. 7 again), the Pb isotopic evolution model routinely used to evaluate Pb isotopic ratios measured today in minerals and rocks being U-Pb dated is that of Stacey and Kramers (1975) (see fig. 8 again). Both models begin with the primordial Pb isotopic composition assumed to be that of the Canyon Diablo iron meteorite’s troilite. However, the Holmes-Houtermans model was superseded by the Stacey and Kramers model, simply because the former was not adequate to explain the Pb isotopic data available from the numerous ongoing research studies. Yet it is abundantly evident that according to Tera (2107a, b) the Stacey and Kramers (1975) Pb evolution model is not fully capable of providing accurate determinations of the isotopic compositions of the common or initial Pb as measured in minerals and rocks today. This is the case even though after 40 years the Stacey and Kramers (1975) model is still used as standard practice in all geochronology laboratories without even consideration of more recently proposed Pb-evolution models. No matter which mass spectrometer systems are used, the Stacey and Kramers (1975) model is still built into all the recommended protocols and software packages within the geochronology community (for example, Horstwood et al. 2016; McLean, Bowring, and Bowring 2011; McLean, Bowring, and Gehrels 2016). Clearly, they are holding onto a failed model because a better model has not yet been developed.
This becomes a major problem for accurately determining absolute U-Pb radioisotope ages. Already built into every U-Pb age determination is the assumption of the isotopic composition of primordial Pb. From that starting condition for the original Pb when the earth formed from the solar nebula, the isotopic composition of the Pb in subsequent minerals and rocks has evolved over time according to their contents of U and Th, and assumed constant radioisotope decay rates. However, various disturbances have occurred to the assumed steady-state evolution of the Pb through earth history. How many such disturbances have occurred varies from region to region, and even within regions, making the task of the geochronologist painstakingly difficult and virtually impossible to unravel the history of the Pb isotopes measured in minerals and rocks today.
Furthermore, when a rock forms from a magma, for example, that magma was first derived via partial melting from a mantle or crustal source. There is never any guarantee that all the U, Th, and Pb isotopes in the source which partially melted were transferred into the melt that became the magma. In fact, partitioning of various elements and their isotopes are known to occur between the source material and the partial melt. Then when the rock crystallizes from the magma the various elements and their isotopes are partitioned into the different mineral phases according to how the various elements and their isotopes fit within the crystal lattices of those minerals based on each element’s ionic radii and charge as well as its chemical properties. Similarly, when a sedimentary or igneous rock is metamorphosed, new mineral phases are usually formed during the transformation of the rock. Again, the various elements and their isotopes are partitioned into those new mineral phases according to how they fit within their crystal lattices based on each element’s ionic radii and charge as well as its chemical properties. What this means is that there is never any guarantee that all the atoms of U, Th, and Pb isotopes in the source or precursor rocks will be transferred into the new rocks that form and their constituent minerals. Thus the common Pb of a region may not become the initial Pb in a rock or mineral inherited from its source within that region.
In the Stacey and Kramers (1975) two-stage Pb isotopic evolution model a major disturbance of the earth occurred at 3.70 Ga (see fig. 8). They identified that as a major differentiation event at that time, based on that being the supposed age of the Amitsoq gneisses of West Greenland. But then they admitted that “marked irregularity in uranium-lead characteristics shows that no single worldwide differentiation event occurred in the early Archean to initiate the second stage of the two-stage model. Therefore, the two-stage concept can only be an approximation” (Stacey and Kramers 1975, 220). Central to the construction of their two-stage model were the Pb isotopic analyses of galena (PbS) from thirteen conformable metal ore deposits of various ages and of 23 feldspars from rocks of various ages. These they plotted on the single-stage Pb isotopic growth curve commencing with the assumed primordial Pb isotopic composition on a 206Pb/204Pb versus 207Pb/204Pb diagram. Their objective was to match these galena and feldspar Pb isotopic data points which plotted close to that growth curve to isochrons linking their Pb isotopic evolution to starting from that 3.70 Ga differentiation event. However, this whole process was dependent on using the previously determined accepted U-Pb ages for the galenas and feldspars. So again, circular reasoning was used in the construction of this two-stage Pb isotopic evolution model assuming a deep time history, which was then used to assign deep time model ages to those galenas and feldspars. In any case, as already quoted, Stacey and Kramers (1975) considered their model “can only be an approximation.”
All of that has been overturned by the very recent work of Tera (2017a, b). He demonstrated that reality presents a far more complicated picture. Pb isotopic evolution is likely different for each and every region around the globe, and likely varies within every region. Furthermore, even then there is a lack of independent evidence to relate the Pb-Pb isochrons derived from Pb isotopic analyses of today’s surface rocks within a region, and used to determine the common Pb component of that region, to an inferred reservoir from which the region’s rocks were supposedly all sourced. Instead, the isochrons whose slopes are said to be equal to the in situ decay ratios of *207Pb/206Pb which yields their supposed ages may simply be mixing lines due to elemental fractionation between two end-members—the initial Pb isotopic compositions and the in situ decay *207Pb/206Pb ratios (the y-axis intercepts on the 204Pb/206Pb versus 207Pb/206Pb plot). Consequently, associating Pb-Pb ages with the determined initial Pb isotopic compositions may not always be meaningful.
According to Tera (2017a, b), in general the initial Pb isotopic composition of a terrestrial terrain evolved in more than one stage. He maintained that a method for determination of all the stages of initial Pb isotopic evolution is necessary for complete outlining of the earth’s history. But he admitted such a method does not exist, and may not even be possible to produce! However, he still claimed resolution to two and three stages (depending on certain constraints) is possible, with the dates of these stages being “congruent approximations.” Yet when he applied his methodology to resolve the first two stages of what he considered a well-studied and dated region, the determined duration of the first stage had to be only an upper limit in a multistage solution whose meaning “remains subject to qualitative speculations”! And the ambiguity in his evolutionary speculations underlined to him the crucial need for a methodology to resolve the initial Pb composition to more of its multi-stages. This surely begs the question—how then can geochronologists be so sure their instrumentally measured Pb isotope ratios can be used in their processing methodologies and statistical treatment protocols to so precisely determine the common or initial Pb in their samples being U-Pb dated, when the very Pb isotopic evolution model they continue to use is more than 40 years old, and has been superseded by a methodology that results in “congruent approximations” and “qualitative speculations” in an assumed deep time evolutionary history? Furthermore, if they cannot with certainty determine the common Pb content of their measured Pb isotopic ratios, how can they so confidently extol their calculated U-Pb radioisotope ages as so accurate and absolute?
Mantle and crustal reservoirs
As already stated earlier, it was in large part the discovery that modern lavas, particularly oceanic basalts, yielded old radioisotope “ages” (Gast, Tilton, and Hedge 1964) which led to the recognition and definition of geochemical reservoirs in the mantle where these lavas had been sourced (Snelling 2000). Zindler and Hart (1986) delineated five end-member compositions in the mantle by which a variety of mixing processes were regarded as capable of explaining all the Sr-Nd-Pb isotope geochemical data pertaining to mid-ocean ridge and ocean-island basalts around the globe. Similarly, Taylor, Jones, and Moorbath (1984) had recognized three isotopic reservoirs in the continental crust, also characterized with respect to Sr-Nd-Pb isotopes. What these mantle and crustal geochemical reservoirs (whose isotopic characteristics were listed by Rollinson 1993) actually represent is still somewhat uncertain and the subject of ongoing investigations. And it must be noted that it is not yet clear exactly where these geochemical (Pb isotopic) reservoirs are located within the earth.
These geochemical reservoirs and their isotopic compositions have also been linked in mantle-crust dynamics models to the processes of plate tectonics through a deep time earth history in order to solve what had become known as the “Pb isotope paradox” (for example, Albarède 1998; Brandenburg et al. 2008; Castillo 2016; Doe and Zartman 1979; Kramers and Tolstikhin 1997; Kumari, Paul, and Stracke 2016; Murphy, Kamber, and Collerson 2003; Phipps Morgan and Morgan 1999; van Keken, Hauri, and Ballentine 2002; White 2015; Zartman and Haines 1988; Zindler and Hart 1986). It is envisaged that complex mixing has occurred through time as the upper and lower mantle have been stirred by the subduction of plates, convection, and the ascent of plumes. Crustal growth has thus also resulted from repeated extractions of partial melts from the upper mantle and lower crust, and has also involved mixing of the various mantle and crustal isotopic reservoirs.
However, as already explained, this multiple mixing and these repeated extractions have resulted in different crustal terrains and regions at the earth’s surface, each having its own individualized multistage evolution of its Pb isotopes. Thus Tera (2017a, b) found that even for a well-dated region its own individualized multi-stage Pb isotopic evolutionary history was unresolvable. And no wonder, because we simply do not know how and where these geochemical/isotopic reservoirs are located inside the earth, and the indications are from the surface rocks that the earth has experienced a very complex history which we may never be able to fully unravel. Furthermore, it is impossible to quantify the mixing and/or extraction and fractionation of the various isotopes at each stage, which then impacts the Pb isotopic evolution in the next stage.
Recognizing the difficulties of modelling the complexities in the available geochemical and isotopic data in assigning them to reservoirs whose locations could be pinpointed, Phipps Morgan and Morgan (1999) tried to simplify the task by just focussing the effects on the mantle of the production of mid-ocean ridge basalts (MORBs) and ocean-island basalts (OIBs). Thus, they proposed a model for mantle evolution in which a sequence of hotspot and ridge upwellings had melted the mantle to make hotspot and mid-ocean ridge basalts and their left-behind residues, while plate subduction had recycled and stirred all of these differentiation products back into the mantle. After the assumed billions of years, they claimed that this process would have mixed various “plums” of incompatible-element rich veins within a matrix made of residues of partial melting that had been depleted in incompatible elements. Phipps Morgan and Morgan (1999) proposed that the mantle flowed upward and melted in a two-stage process. They maintained that the observed geochemical and isotopic contrasts between MORBs and OIBs could thus have been produced by a recipe which assumes that throughout earth history these two sequential stages of deep plume and shallower ridge melting would both have created and reprocessed the “plums” and residues that make up the present-day mantle. However, rather than stratified mantle convection, with a depleted upper mantle MORB reservoir and an undepleted lower mantle OIB reservoir (fig. 9), their model involved convection of the whole mantle that is a mixture of “plums” and residues, which they suggested also seems consistent with the geophysical evidence of significant past flow having occurred between the upper and lower mantle (fig. 10).
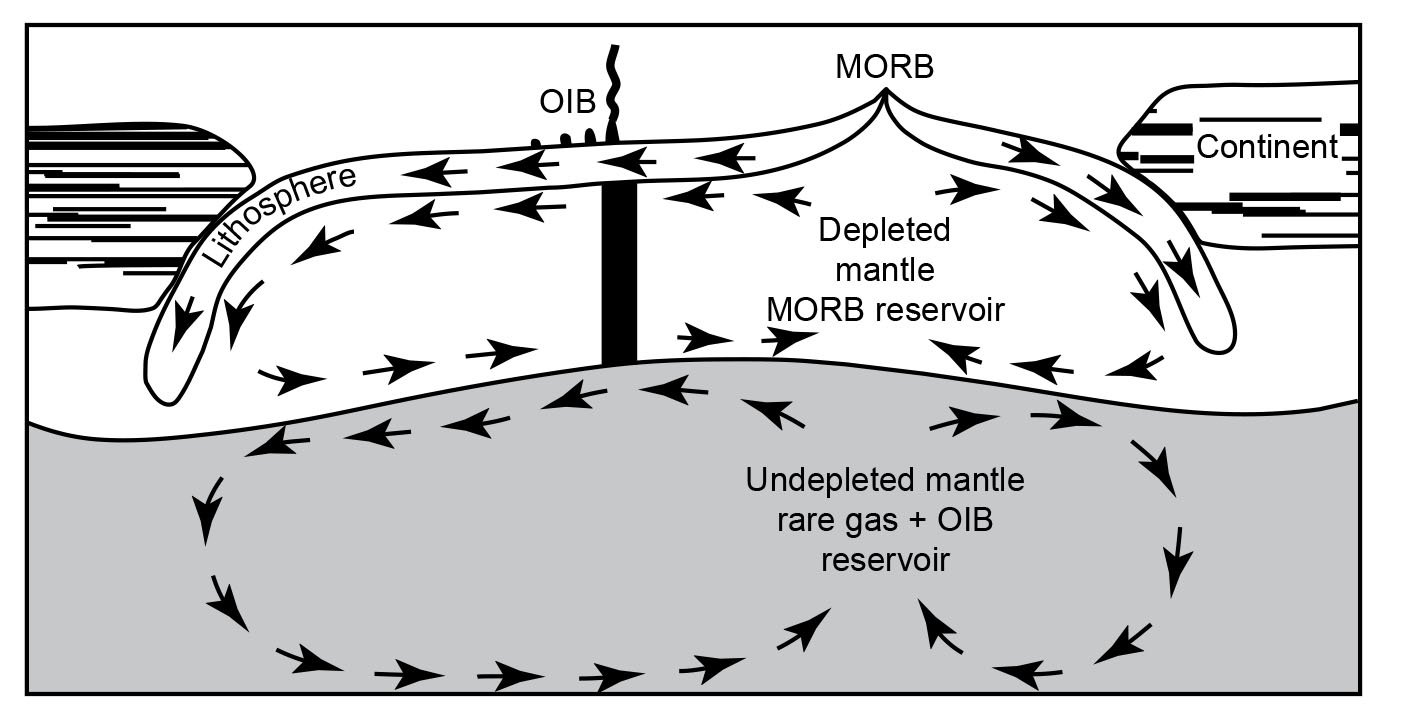
Fig. 9. Diagram showing a model of stratified mantle convection that is consistent with the observed geochemistry and isotopic composition of MORBs and continental crust (after Phipps Morgan and Morgan 1999). This model explained the observed geochemical differences between MORBs and OIBs and between MORBs and the continental crust, as well as the observed isotopic depletion of MORBs and isotopic enrichment of the continental crust. However, it does not seem to be consistent with geophysical evidence for significant past flow between the upper and lower mantle.
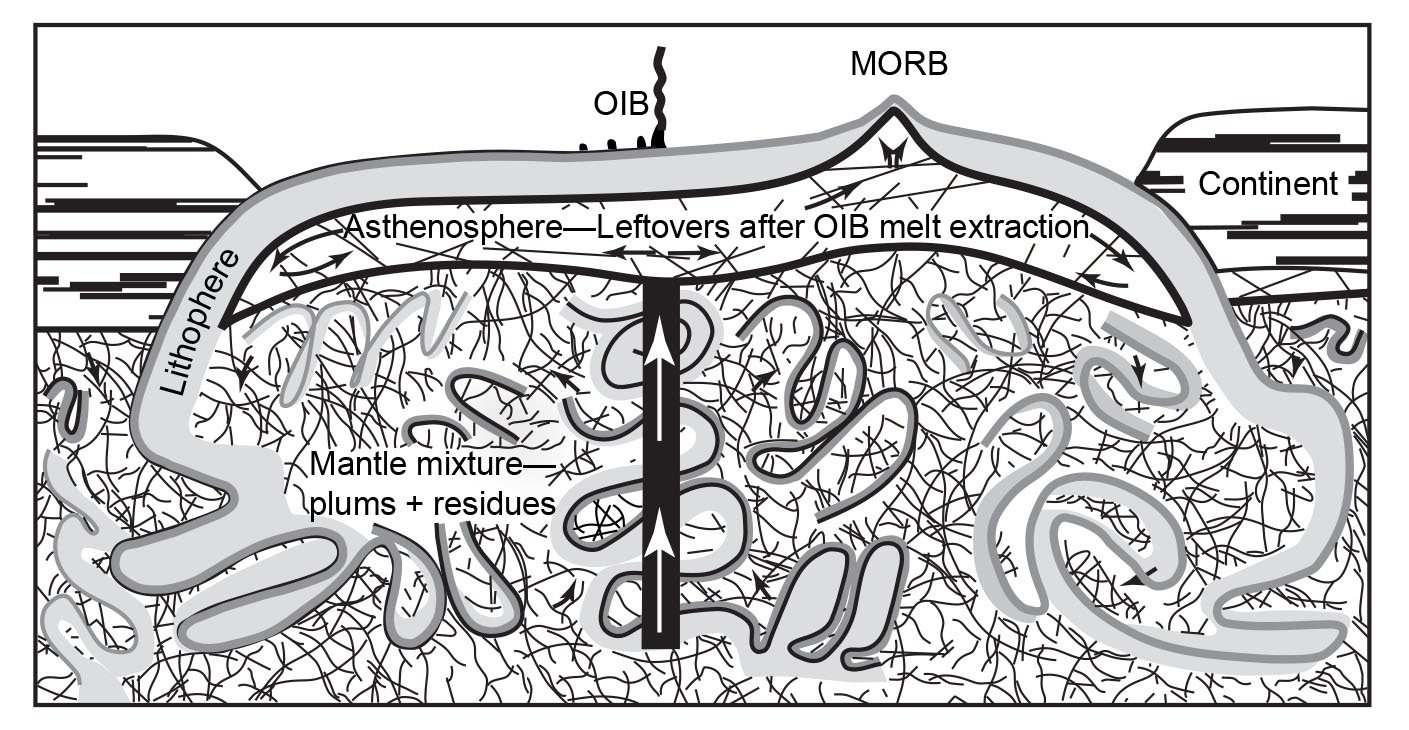
Fig. 10. Diagram showing the alternative model of whole mantle convection that appears to be more consistent with both geochemical/isotopic and geophysical evidence (after Phipps Morgan and Morgan 1999). In this model the mantle is a “plum-pudding” mixture of recycled basalts and continental sediments, rich in incompatible elements, that has been stirred by convection into recycled residues from plume and ridge melt-extraction and surviving “primitive” (original primordial) mantle. The asthenosphere “MORB-source” is derived from typical “plum-pudding” mantle by partial melting of an upwelling plume—the plume partial melts become OIBs, while the depleted leftovers from plume-melting pond beneath the lithosphere to supply the asthenosphere. When this depleted asthenosphere upwelled and partially melted a second time beneath a ridge, its partial melt became a MORB.
That the geochemical/isotopic reservoirs inside the mantle and crust are very complex, and their locations and character are unable to be fully identified and resolved, is still the case, as admitted by Tera (2017a, b). Thus it becomes virtually impossible to be certain that the contribution of common or initial Pb to the Pb isotopic ratios measured in minerals and rocks today can be adequately and accurately identified and quantified. Consequently, it is surely premature for geochronologists to be so dogmatic that they can U-Pb date today’s surface rocks with such accuracy to declare their results as absolute ages.
Effects on U-Pb and Pb-Pb ages
Thus, the isotopic composition of the common or initial Pb component cannot be adequately and accurately resolved within the Pb isotopic ratios measured in minerals and rocks today. Yet knowing the common or initial Pb isotopic composition is critical and essential for determining accurate and meaningful U-Pb and Pb-Pb ages, as can be easily demonstrated.
Equations (6) and (7) provide the 206Pb and 207Pb model ages. Note that the initial values of the 206Pb/204Pb and 207Pb/204Pb ratios need to be known separately from the 206Pb/204Pb and 207Pb/204Pb ratios measured in the mineral or rock. Indeed, as stated by Faure and Mensing (2005, 218–219) in their list of conditions that need to be satisfied for the two model ages to agree with each other, the correct values for the initial Pb isotope ratios need to be used in these equations. It is usually claimed that the choice of the initial Pb isotope ratios would seem to only be a problem for dating rocks and minerals that have low U/Pb ratios and additionally are young, and that the numerical values of the initial Pb isotope ratios do not appear to significantly affect the calculated U-Pb ages of Precambrian rocks and minerals having high U/Pb ratios, because their present Pb isotope ratios in most cases reach large values. However, the claim regarding Precambrian rocks and minerals is made without definitive proof that not being able to prescribe the initial Pb isotope ratios is not a problem. To the contrary, the choice made by Tera (2017a, b) of a well-dated region on which to test his methodology for determining the initial Pb composition was a Precambrian terrain. And then he still concluded that he could not resolve what the common or initial Pb composition was as distinct from the primordial Pb, except for the presumed first stage of the Pb isotopic evolution which still remained a “congruent approximation” subject to “qualitative speculations”!
Equation (9) provides the 207Pb-206Pb model age, which again is dependent on knowing the values of the initial 206Pb/204Pb and 207Pb/204Pb ratios. They also appear in the derivative equation (10). So again, if the values of these initial Pb isotope ratios cannot be accurately determined, then by merely depending on the measured 206Pb/204Pb and 207Pb/204Pb ratios being essentially the same as the radiogenic 206Pb/204Pb and 207Pb/204Pb ratios, and thus effectively ignoring the undetermined values for the initial 206Pb/204Pb and 207Pb/204Pb ratios, does not guarantee that the 207Pb-206Pb model age is accurate or absolute. Indeed, even if it is argued that the 206Pb, 207Pb, and 207Pb-206Pb model ages all agree with one another (are concordant), that is really only because the values of the initial 206Pb/204Pb and 207Pb/204Pb ratios used in the relevant equations are all the same, in spite of them not having been accurately determined. So it does not make these model ages any more accurate or absolute simply because they are concordant! They all suffer from the same systematic problem— the values for the initial Pb isotope ratios cannot be accurately known.
Equations (12) and (13) are used to construct the Wetherill concordia on which the measured Pb isotope data of samples are plotted, and based on them a discordia age for the rock is determined graphically (fig. 3). Again, knowing the values for the initial 206Pb/204Pb and 207Pb/204Pb ratios are essential in these equations. Furthermore, ignoring them because they cannot be accurately determined does not make the concordia curve any more accurate as the loci of all the concordant 206Pb and 207Pb model ages, simply because those 206Pb and 207Pb model ages have the same systematic error making their concordancy systematically wrong! So the discordia ages derived from intersections with this Wetherill concordia curve will be no more accurate or absolute than the 206Pb and 207Pb model ages used to derive it.
Equations (16) and (17) are derived from equations (14) and (15), and are then used to construct the Tera-Wasserburg concordia. On this concordia diagram the measured 207Pb/206Pb and 238U/206Pb ratios of samples are plotted, and based on them a discordia age for the rock or mineral is determined graphically (fig. 4). The slope of the discordia is given in equation (19). The values for the initial 206Pb and 207Pb values are needed in equations (14) and (15) respectively, but as already noted the mass spectrometers used for U-Pb isotopic analyses do not (and cannot) measure these absolute quantities with any certainty, but can only measure the present-day isotopic ratios of the samples. Furthermore, the initial values of those isotope ratios cannot be determined with certainty from the measured present-day isotope ratios, as all 206Pb and 207Pb atoms are identical whether they were in the samples initially or have been added by subsequent radioactive decay of 238U and 235U respectively. Similarly, the value of the initial 207Pb/206Pb ratio is essential to equation (19), but it likewise cannot be determined with any certainty from the sample’s measured Pb isotope ratios. Thus, to determine the age of a lunar basalt, Tera and Wasserburg (1972) had to assume a value for the initial 207Pb/206Pb ratio to obtain a discordia (fig. 5). It should also be noted that often one of the discordia intercepts with the concordia curve (usually the lower intercept) is either unknown or meaningless. Surely that is to be expected if the initial isotope ratios on which this whole concordia methodology depends cannot be known with any certainty, thus rendering any obtained dates suspect and certainly not absolute.
Of all the U-Pb ages obtainable from the U-Pb isotopic data for a suite of rock or mineral samples, the Pb-Pb isochron age is usually regarded as the most reliable. Equation (20) defines how a Pb-Pb isochron is constructed, while equation (21) is for the slope of the isochron which yields its Pb-Pb isochron age. The values of the initial 206Pb/204Pb and 207Pb/204Pb ratios are essential in equation (20) so that the radiogenic 207Pb/206Pb ratio can be determined, without which no meaningful line can be plotted that will be a valid isochron yielding a valid isochron age. Indeed, Faure and Mensing (2005, 221) specifically state that age determinations by this Pb-Pb isochron method depend on the assumption that all samples that define the isochron had the same initial Pb isotope ratios. How can that even be a valid assumption when it is known that different elements are partitioned between different minerals in the same rock as they crystallize, so that the same initial Pb in a magma, for example, will not likely get evenly distributed into the resultant mineral crystals in easily quantifiable proportions? Similarly, different rock samples from the same rock unit are not guaranteed to have the same amounts of initial Pb isotopes in them, even in proportion to each of their total Pb contents. And again, as has already been emphasized, it is not possible to distinguish between initial and radiogenic-derived Pb isotopes based on the measured Pb isotope ratios. So, without being able to reliably determine the initial 206Pb/204Pb and 207Pb/204Pb ratios, it is never possible to be sure the derived isochron age is even a true absolute age.
It might be argued that the initial Pb isotopic composition can be determined on the Pb-Pb isochron diagram from the 207Pb/204Pb intercept value on the y-axis where 206Pb/204Pb = 0 on the x-axis. However, not even primordial Pb has a 206Pb/204Pb value of 0. Nor does it make any sense if the Pb-Pb-isochron intersects the 207Pb/204Pb y-axis at zero, or intersects the 206Pb/204Pb x-axis at some value when 207Pb/204Pb = 0 on the y-axis. None of those scenarios for an initial Pb isotopic composition matches that for primordial Pb, and common Pb is supposed to always contain all four stable Pb isotopes, not zero of some. Thus, the Pb-Pb isochron cannot be used to determine the initial Pb isotopic composition, which is crucial to be known if the Pb-Pb isochron age is the true age, per equation (20) for deriving the Pb-Pb isochron.
Equation (28) is the basis for yielding the Pb-Pb isochron age of samples containing only common Pb, for example, minerals that contain negligible U or Th and thus their Pb content was not produced by in situ radioisotope decay of U or Th after the mineral formed. However, not only does equation (28) require that the initial 206Pb/204Pb and 207Pb/204Pb ratios be known, but also the age of the earth must be known. Yet the age of the earth was determined by assuming the meteorites and the earth formed at the same time from the solar nebula by uniformitarian evolutionary processes with the same primordial Pb isotopic composition, and on the basis of the Pb isotopic compositions of galena samples of assumed “known ages.” These are unproven assumptions. And again, the initial 206Pb/204Pb and 207Pb/204Pb ratios simply cannot be accurately determined using mass spectrometers measuring the 206Pb/204Pb and 207Pb/204Pb ratios in the minerals today, because the mass spectrometers cannot distinguish between the initial 206Pb and 207Pb atoms and the identical later-formed 206Pb and 207Pb atoms. Thus, common Pb-Pb isochron dating cannot provide accurate absolute dates for common Pb minerals.
Furthermore, Tera (2017a, b) warned that there is a lack of independent evidence to relate the Pb-Pb isochrons and their associated ages derived from Pb isotopic analyses of today’s surface rocks in a region to their source. Instead, the isochrons whose slopes are said to be equal to the in situ decay Pb/Pb ratios which yield their supposed ages may simply be mixing lines due to elemental fractionation between two end-members—the initial Pb isotopic compositions and the in situ decay Pb/Pb ratios. And if we cannot determine with any certainty what the initial Pb compositions were, then we cannot be sure whether the straight lines on a Pb isotopes plot are true isochron ages, or whether they are simply mixing lines with no meaningful ages associated with them.
Significance within the biblical model of earth history
The biblical model of earth history has a radically different timeframe than the conventional uniformitarian/evolutionary model. The timeframe over which God created everything in the universe was six literal ordinary timespan days (Genesis 1 and Exodus 20:11). And that occurred only about 6000 or so years ago, based on the genealogies in Genesis 5 and 11 and the timeframe of the patriarchs and their descendants in the history of Israel, effectively the genealogy of Jesus Christ (Matthew 1:1–17 and Luke 3:23–38). So there is no room for cosmic or geologic evolution over billions of years of radioisotope decay or Pb isotopic evolution.
From the foregoing discussion one conclusion can be validly made. It is entirely reasonable scientifically for biblical Christians to believe that when God brought the earth into existence at the beginning by fiat creation (rather than by cosmic evolution over billions of years), He gave the earth an initial Pb isotopic composition which included all four stable Pb isotopes. However, in the biblical model of earth history it is not necessary to assume that the earth’s primordial Pb isotopic composition was that of the Canyon Diablo iron meteorite’s troilite. To the contrary, the evolutionary model starts with the sun forming first and the earth and solar system formed subsequently out of the sun, whereas the biblical account of God creating the universe starts with Him creating the earth and producing dry land on it covered with plants and surrounded by seas before He created the sun, moon, and stars. Though not mentioned specifically, presumably the other planets, moons, and asteroids of the solar system were also created on Day 4, three days after the earth was created.
Furthermore, it is obvious that when God created the earth with its initial endowment of all four stable Pb isotopes, none of those Pb atoms had been derived by radioactive decay from U or Th isotopes. And since that initial or primordial Pb isotopic endowment God gave the earth was not that of the Canyon Diablo iron meteorite’s troilite, the starting point in Pb isotopic evolution models on the created earth could be very different to that assumed by evolutionists. But what that created primordial Pb isotopic endowment might have been we obviously cannot be sure. Because we do not even have a basis to develop such a model, we are left to speculate and propose various combinations that could then be used as the starting points of Pb isotopic evolution models to test whether they fit the Pb isotopic data of negligible-U- or -Th-bearing common Pb minerals such as ore deposit galenas according to their relative ages. For example, could God have created equal amounts of the four stable Pb isotopes in the primordial Pb of the earth? If so, then the isotopic composition of the primordial Pb would have had 206Pb/204Pb, 207Pb/204Pb and 208Pb/204Pb ratios all equal to 1, compared to the very much different values of the Pb in the Canyon Diablo iron meteorite’s troilite (table 3).
The sequence of events when subsequent mixing of Pb isotopes would have occurred is also radically different. The scriptural record only states that when God created the earth on Day 1 of the Creation Week it was covered in water. We are not told whether there was anything under the waters, but it seems logical to assume that God also created the differentiated internal structure of core, mantle, and a crust on Day 1, because on Day 3 He created the dry land and put soil and plants on its surface. Alternately it could be suggested that He formed all the elements from the water on Day 1 and then fashioned them into the core, mantle, and crust on Day 3. However, that scenario is less tenable in light of the evidence in the geologic record of crustal rocks and fossilized microbial life back close to the time of the earth’s formation (Purdom and Snelling 2013). How God reorganized the earth’s original crust and surface on Day 3 is not described, but it is reasonable to suggest earth movements occurred to uplift sections of that crust. If those earth movements were accompanied by any melting and subsequent cooling and crystallization, some redistribution of Pb isotopes may have occurred during that Day 3 “Great Upheaval”.
Throughout the remainder of the Creation Week the earth’s crust would have been relatively stable so as not to disrupt the plants and animals living on its surface in a world God pronounced very good. Exactly what changes took place soon thereafter at the time of the Fall, when God cursed the ground which brought forth thorns and thistles (Genesis 3:17–18), we are not told. However, if the Fall and the Curse brought into being bad things, then perhaps when the ground was cursed radioactive decay was triggered so that U and Th isotopes became unstable and started to decay. Some creationists prefer that option over radioactive decay already happening during the Creation Week, from the time when God had created the parent radioisotopes. It is not so much that the “decay” process is bad, because it is effectively only the transmutations of parent atoms into daughter atoms, the latter being just as good as the former. Rather, it is the radiation released during the decay process which is harmful to plant and animal life. So for the world of the Creation Week to be very good, there must have been no harmful radiation and thus no radioactive decay in rocks and soils at the earth’s surface, or all the radioisotopes were only deep inside the earth in the mantle and core where the harmful radiation from their radioactive decay would be absorbed.
Therefore, if radioactive decay was always present inside the earth from the initial creation, then Pb isotopic evolution would have commenced simultaneously as U and Th decay added Pb isotopes to the primordial (created) Pb isotopic composition from Day 1 onwards. Furthermore, the earth movements and reorganization of the earth’s crust to make the dry land during the “Great Upheaval” on Day 3 would have caused a major disturbance that redistributed various elements and their isotopes, including U, Th, and Pb, thus greatly changing the isotopic evolution of Pb. On the other hand, if radioactive decay was not triggered until the Curse at an unspecified time after the Creation Week, then any major Pb isotopic evolution due to U and Th decay adding Pb daughter isotopes to the primordial (created) Pb isotopic composition would only have occurred from the Curse onwards.
In either case, though, it is argued that the earth’s oldest rocks do not record up to 4 billion or more years of radioactive decay. Apart from the radioisotope ratios there is no physical evidence, such as radiohalos and fission tracks (Snelling 2005a, b), of billions of years’ worth of radioisotope decay in the earth’s earliest rocks. We simply do not know how much daughter isotopes were in the first (created) rocks and thus what were the initial isotope ratios. Even the same applies to daughter helium from U and Th decay, as minerals and rocks are known to inherit helium atoms when they form (for example, in volcanic rocks). We don’t know what the earth’s initial endowment of helium was, so we cannot assume the helium in the earliest rocks all came from U and Th decay. Besides, apart from the Curse, after the end of the Creation Week there was no significant event during which huge amounts of accelerated nuclear decay could have occurred, except during the Flood. Indeed, we have the physical evidence of radiohalos and fission tracks for that accelerated nuclear decay during the year-long Flood and its aftermath (Snelling 2005a, b).
So the next major event in the biblical model of earth history was the global Flood cataclysm that totally reshaped the earth, including the mantle and especially the crust. The assumption of uniformitarian process rates for the geologic processes involved over some 600 million years or so is rejected. Instead, all the geological changes occurred in the year of the Flood and the subsequent decades of its aftermath as the earth recovered from that convulsion. All the postulated melting and mixing in the conventional model of geochemical/isotopic reservoirs inside the mantle and crust during that supposed 600 million or so million years could conceivably still have been accomplished by catastrophic plate tectonics within this young-earth timeframe (Austin et al. 1994; Baumgardner 2003; Snelling 2009). If the Flood cataclysm was also accompanied by grossly accelerated nuclear decay as postulated by Vardiman, Snelling, and Chaffin (2005), then 600 or so million years’ worth of daughter Pb isotopes and portions thereof were sequentially added to crustal minerals and rocks during the Flood year, drastically changing their Pb isotopic compositions which uniformitarians interpret as great apparent ages.
The net outcome is the Pb isotopic ratios measured in minerals and rocks today. The conventional uniformitarian approach to their interpretation postulates a steady-state Pb isotopic evolution in the earth’s minerals and rocks from the earth’s assumed primordial Pb isotopic composition through an assumed long history of disturbances over 4.5 billion of years of assumed radioisotope decay at today’s slow rates. Yet uniformitarian geochronologists cannot distinguish with certainty from today’s measured Pb isotopic ratios what portion of them represents the isotopic ratios of the initial Pb inherited by today’s minerals and rocks when they formed from the common Pb in the mantle and crustal geochemical/isotopic reservoirs that seem to exist inside the earth. And what portion are the daughter Pb isotopic ratios resulting from in situ U and Th decay after the minerals and rocks formed? Furthermore, the compositions of those geochemical/ isotopic reservoirs have themselves been changed over time by disturbances stirring and mixing them, and by extraction from them of partial melts. Those uncertainties make it impossible to be dogmatic in asserting that today’s measured Pb isotopic ratios provide absolute ages of millions and billions of years for today’s minerals and rocks.
The earth’s deep time history has only been assumed, not proven. To the contrary, the infallible divine eyewitness account in the Scriptures provides a reliable framework for the earth’s short history of only 6000 or so years marked by the progressive supernatural creation processes during the days of the Creation Week, the supernatural Curse at the Fall, and the cataclysmic total reshaping of the earth in the year-long Flood. And the multiple lines of evidence consistent with grossly accelerated nuclear decay at least during the Flood (Vardiman, Snelling, and Chaffin 2005) renders any claims of absolute U-Pb ages in the millions and billions of years totally without any substantiation.
The challenge then is to now build an alternative model for the earth’s Pb isotopic evolution, starting with an assumed realistic created primordial Pb isotopic endowment at the earth’s creation on Day 1. The isotopic redistribution and mixing effects of the subsequent supernatural events during the Creation Week’s days would need to then be added to the model. Then depending on when it is assumed radioisotope decay began, the model would incorporate the steady accumulation of daughter Pb isotopes that resulted from the likely slow U and Th decay which occurred in the pre-Flood earth. And finally, the model would then add to the Pb isotopic evolution the grossly accelerated radioisotope decay during the Flood cataclysm and its immediate aftermath, when numerous mineral and rock formation cycles occurred to build the earth’s present crustal configuration. Such a model’s “success” would then be judged on how accurately it quantified the various contributions to, and thus matched, the measured Pb isotopic ratios in today’s minerals and rocks. Quite clearly there will likely be needed numerous iterations in this modelling process with different assumed initial (created) primordial Pb isotope endowments, until the endowment which best fits today’s measured Pb isotope ratios is achieved, after the subsequent additions of Pb isotopes during the biblical framework and elapsed timescale for the earth’s history.
Conclusions
Uniformitarians assume that when the earth formed it already had an initial Pb isotopic endowment, which is referred to as primordial Pb. That has been determined as the Pb isotopic composition of troilite in the Canyon Diablo iron meteorite. Subsequent radioactive decay of U and Th has added Pb isotopes to the primordial Pb inside the earth. Melting and mixing in the mantle and crust through the earth’s history have redistributed U, Th, and Pb isotopes to produce mantle and crustal geochemical reservoirs with different common Pb isotopic compositions. At various times, partial melts have been extracted from these common Pb reservoirs to produce new crustal rocks which inherited some, or all, of the Pb isotopes obtained from their sources. Subsequent U and Th decay in these new crustal rocks added more Pb isotopes to their initial inherited Pb isotopic composition. Eventually exposed at the earth’s surface, the Pb isotopic ratios in these minerals and rocks are measured today and used to U-Pb date them.
However, because the respective inherited initial and radiogenic Pb atoms are identical, how can the primordial, inherited (initial) and radiogenic Pb atoms in a mineral or rock today be distinguished from one another? There is no question that due to the sophistication of todays’ analytical equipment, the Pb isotope ratios in minerals and rocks can be accurately measured. Yet, to determine the age of a mineral or rock requires the counting of only the radiogenic (daughter) Pb atoms which have been produced by U and Th decay in it since it formed, assuming constant rates of U and Th decay at today’s measured slow rates. So, unless those daughter Pb atoms can be distinguished from the initial inherited Pb atoms, it is not possible to be certain of the mineral or rock’s age using the U-Pb dating method.
The mass spectrometers used to analyse a rock’s Pb isotopes only measure what its Pb isotope ratios are today. And the absolute quantities of each of the Pb isotopes are difficult to determine, so only the Pb isotopic ratios are measured. Thus, numerous methods have been devised to determine the initial Pb isotopic composition from the measured Pb isotope ratios as distinct from the radiogenic (daughter) Pb atoms to use the latter to calculate the rock’s U-Pb age. It is claimed that the initial Pb can thus be routinely determined by these methods and can thus be eliminated from all U-Pb age calculations. However, all these methods involve assumptions that cannot be proven. Zircon does incorporate initial Pb when it crystallizes. The amount of 204Pb cannot be measured independently and accurately. It cannot be demonstrated that the initial Pb only consisted of 204Pb atoms. It cannot be proven that the Pb in apparently cogenetic U- or Th-free minerals is only initial Pb, and that it is identical to the initial Pb in the mineral (zircon) being dated. Nor can the measured 206Pb, 207Pb, and 208Pb isotope ratios be used to somehow decide what proportions of them are the initial Pb without recourse to unprovable assumptions about the mineral or rock’s history or their interpreted U-Th-Pb ages within an assumed deep time history.
However, the ultimate foundation of this U-Pb dating methodology is the assumption that the earth formed out of the solar nebula and that its primordial Pb isotopic endowment was identical to that in the troilite of the Canyon Diablo iron meteorite. This is a very shaky assumption, as one would naively hypothesize that the heavier metals, like Pb, would migrate to the inner planets rather than the asteroids which appear to have parented most of the meteorites, especially iron meteorites which are believed to be derived from the cores of asteroids found today in the asteroid belt beyond Mars. From a biblical perspective, though, the earth was created by God on Day 1 of the Creation Week before the sun and the rest of the solar system were created on Day 4, all only about 6000 or so years ago. Yet the earth would still have had an initial Pb isotopic endowment created by God. Once radioactive decay of U and Th started after creation, radiogenic Pb isotopes were added inside the earth. But then the catastrophic plate tectonics during the year-long Flood stirred the mantle and via partial melting added many new rocks to the crust. These new rocks rapidly accumulated more Pb isotopes due to the concurrent accelerated radioactive decay of U and Th in them during the Flood and its aftermath.
Therefore, without being able to unequivocally distinguish the daughter Pb atoms produced by in situ U and Th decay from the initial Pb atoms in a mineral or rock, it is impossible to determine their absolute U-Pb radioisotope ages. All the unprovable assumptions ultimately depend on an assumed deep time history. Its rejection is recognized as fatal to the earth’s claimed age of billions of years. There is thus no impediment to accepting and using the Bible’s account of Creation and the Flood as a reliable framework for unravelling the history of the earth and the Pb isotopes found in its minerals and rocks.
Acknowledgments
The three reviewers are acknowledged for their very helpful comments and input, though the final content of this paper is solely my responsibility. Our production assistant Laurel Hemmings is also especially thanked for her painstaking work in preparing this paper for publication.
References
Ahrens, L. H. 1955. “Implications of the Rhodesian Age Pattern.” Geochimica et Cosmochimica Acta 8 (1–2): 1–15.
Albarède, F. 1998. “Time-Dependent Models of U-Th-He and K-Ar Evolution and the Layering of Mantle Convection.” Chemical Geology 145 (3–4): 413–429.
Amelin, Y., J. Connelly, R. E. Zartman, J. H. Chen, C. Göpel, and L. A. Neymark. 2009. “Modern U-Pb Chronometry of Meteorites: Advancing to Higher Time Resolution Reveals New Problems.” Geochimica et Cosmochimica Acta 73 (17): 5212–5223.
Andersen, T. 2002. “Correction of Common Lead in U-Pb Analyses That do not Report 204Pb.” Chemical Geology 192 (1–2): 59–79.
Ashton, F. W. 1919. “A Positive Ray Spectrograph.” Philosophical Magazine 38 (series 6): 707–714.
Ashton, F. W. 1927. “The Constitution of Ordinary Lead.” Nature 120: 224.
Austin, S. A., J. R. Baumgardner, D. R. Humphreys, A. A. Snelling, L. Vardiman, and K. P. Wise. 1994. “Catastrophic Plate Tectonics: A Global Flood Model of Earth History.” In Proceedings of the Third International Conference on Creationism. Edited by R. E. Walsh, 609–621. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Baumgardner, J. R. 2003. “Catastrophic Plate Tectonics: The Physics Behind the Genesis Flood.” In Proceedings of the Fifth International Conference on Creationism. Edited by R. L. Ivey, Jr., 113–126. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Begemann, F., K. R. Ludwig, G. W. Lugmair, K. Min, L. E. Nyquist, P. J. Patchett, P. R. Renne, C.-Y. Shih, I. M. Villa, and R. J. Walker. 2001. “Call for an Improved Set of Decay Constants for Geochronological Use.” Geochimica et Cosmochimica Acta 65 (1): 111–121.
Boehnke, P., and T. M. Harrison. 2014. “A Meta-Analysis of Geochronologically Relevant Half-Lives: What’s the Best Decay Constant?” International Geology Review 56 (7): 905–914.
Boltwood, B. B. 1907. “On the Ultimate Disintegration Products of the Radioactive Elements.” American Journal of Science 23 (4): 77–88.
Bowring, S. A., D. H. Erwin, Y. G. Yin, M. W. Martin, K. Davidek, and W. Wang. 1998. “U/Pb Zircon Geochronology and the Tempo of the End-Permian Mass Extinction.” Science 280: 1039–1045.
Bowring, S. A., and M. D. Schmitz. 2003. “High-Precision U-Pb Zircon Geochronology and the Stratigraphic Record.” In Zircon. Edited by J. M Hanchar and P. W. O. Hoskin, Reviews in Mineralogy and Geochemistry, vol. 53, 305–326. Washington DC: Mineralogical Society of America.
Brandenburg, J. P., E. H. Hauri, P. E. van Keken, and C. J. Ballentine. 2008. “A Multiple-System Study of the Geochemical Evolution of the Mantle with Force-Balanced Plates and Thermochemical Effects.” Earth and Planetary Science Letters 276: 1–13.
Brennecka, G. A., and M. Wadhwa. 2012. “Uranium Isotope Compositions of the Basaltic Angrite Meteorites and the Chronological Implications for the Early Solar System.” Proceedings of the National Academy of Sciences USA 109 (24): 9299–9303.
Castillo, P. R. 2016. “A Proposed New Approach and Unified Solution to Old Pb Paradoxes.” Lithos (252–253): 32–40.
Chen, J. H., and G. J. Wasserburg. 1983. “The Least Radiogenic Pb in Meteorites.” Lunar and Planetary Science Conference 14: 103–104.
Chew, D. M., J. A. Petrus, and B. S. Kamber. 2014. “U-Pb LAICPMS Dating Using Accessory Mineral Standards with Variable Common Pb.” Chemical Geology 363: 185–199.
Condon, D. J., B. Schoene, N. M. McLean, S. A. Bowring, and R. R. Parrish. 2015. “Metrology and Traceability of U-Pb Isotope Dilution Geochronology (EARTHTIME Tracer Calibration Part I).” Geochimica et Cosmochimica Acta 164: 464–480.
Dempster, A. J. 1918. “A New Method of Positive Ray Analysis.” Physical Review 11 (4): 316–325.
Dickin, A. P. 2005. Radiogenic Isotope Geology. 2nd ed. Cambridge, United Kingdom: Cambridge University Press.
Doe, B. R., and R. E. Zartman. 1979. “Plumbotectonics.” In Geochemistry of Hydrothermal Ore Deposits. 2nd ed. Edited by H. L. Barnes, 22–70. New York: Wiley.
Faure, G., and T. M. Mensing. 2005. Isotopes: Principles and Applications. 3rd ed. Hoboken, New Jersey: John Wiley & Sons.
Gast, P. W., G. R. Tilton, and C. Hedge. 1964. “Isotopic Composition of Lead and Strontium from Ascension and Gough Islands.” Science 145 (3637): 1181–1184.
Gerling, E. K. 1942. “Age of the Earth According to Radioactivity Data.” Compte Rendus (Doklady) de l’Academie Sciences de l’URSS 34 (9): 259–261.
Goldmann, A., G. Brennecka, J. Noordmann, S. Weyer, and M. Wadhwa. 2015. “The Uranium Isotopic Composition of the Earth and the Solar System.” Geochimica et Cosmochimica Acta 148: 145–158.
Hiess, J., D. J. Condon, N. McLean, and S. R. Noble. 2012. “238U/235U Systematics in Terrestrial Uranium-Bearing Minerals.” Science 335 (6076): 1610–1614.
Holmes, A. 1913. The Age of the Earth. London, United Kingdom: Harper and Brothers.
Holmes, A. 1946. “An Estimate of the Age of the Earth.” Nature 157 (3995): 680–684.
Horstwood, M. S. A., J. Košler, G. Gehrels, S. E. Jackson, N. M. McLean, C. Paton, N. J. Pearson, K. Sircombe, P. Sylvester, P. Vermeesch, J. F. Bowring, D. J. Condon, and B. Schoene. 2016. “Community-Driven Standards for LA-ICP-MS U-(Th-)Pb Geochronology—Uncertainty Propagation, Age Interpretation and Data Reporting.” Geostandards and Geoanalytical Research 40 (3): 311–332.
Housh, T., and S. A. Bowring. 1991. “Lead Isotopic Heterogeneities within Alkali Feldspars: Implications for the Determination of Initial Lead Isotopic Compositions.” Geochimica et Cosmochimica Acta 55 (8): 2309–2316.
Houtermans, F. G. 1946. “Die Isotopenhäufigkeiten im Natürlichen Blei und das Alter des Urans.” Naturwissenschaften 33 (6): 185–186, 219.
Ireland, T. R., and I. S. Williams. 2003. “Considerations in Zircon Geochronology by SIMS.” In Zircon. Edited by J. M. Hanchar and P. W. O. Hoskin, Reviews in Mineralogy and Geochemistry, vol. 53, 215–241. Washington DC: Mineralogical Society of America.
Jackson, S. E., N. J. Pearson, W. L. Griffin, and E. A. Belousova. 2004. “The Application of Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry to In Situ U-Pb Zircon Geochronology.” Chemical Geology 211 (1–2): 47–69.
Kanasewich, E. R. 1968. “The Interpretation of Lead Isotopes and their Geological Significance.” In Radiometric Dating for Geologists. Edited by E. I. Hamilton and R. M. Farquhar, 147–233. New York: Wiley-Interscience.
Košler, J., and P. J. Sylvester. 2003. “Present Trends and the Future of Zircon in Geochronology: Laser Ablation ICPMS.” In Zircon. Edited by J. M Hanchar and P. W. O. Hoskin, Reviews in Mineralogy and Geochemistry, vol. 53, 243–275. Washington DC: Mineralogical Society of America.
Kramers, J. D., and I. N. Tolstikhin. 1997. “Two Terrestrial Lead Isotope Paradoxes, Forward Transport Modelling, Core Formation and the History of the Continental Crust.” Chemical Geology 139 (1–4): 75–110.
Kumari, S., D. Paul, and A. Stracke. 2016. “Open System Models of Isotopic Evolution in Earth’s Silicate Reservoirs: Implications for Crustal Growth and Mantle Heterogeneity.” Geochimica et Cosmochimica Acta 195: 142–157.
Ludwig, K. R. 1980. “Calculation of Uncertainties of U-Pb Isotope Data.” Earth and Planetary Science Letters 46 (2): 212–220.
Ludwig, K. R. 1993. “Pb Dat 1.24: A Computer Program for Processing Raw Pb-U-Th Isotope Data.” USGS Open-File Report 88-542. Washington, D.C.: U.S. Geological Survey.
Ludwig, K. R. 1998. “On the Treatment of Concordant Uranium-Lead Ages.” Geochimica et Cosmochimica Acta 62 (4): 665–676.
Mattinson, J. M. 1994. “A Study of Complex Discordance in Zircons Using Step-Wise Dissolution Techniques.” Contributions to Mineralogy and Petrology 116 (1–2): 117–129.
Mattinson, J. M. 2005. “Zircon U-Pb Chemical Abrasion (‘CATIMS’) Method: Combined Annealing and Multi-Step Partial Dissolution Analysis for Improved Precision and Accuracy of Zircon Ages.” Chemical Geology 220 (1–2): 47–56.
Mattinson, J. M. 2010. “Analysis of the Relative Decay Constants of 235U and 238U by Multi-Step CA-TIMS Measurements of Closed-System Natural Zircon Samples.” Chemical Geology 275 (3–4): 186–198.
McLean, N. M., J. F. Bowring, and S. A. Bowring. 2011. “An Algorithm for U-Pb Isotope Dilution Data Reduction and Uncertainty Propagation.” Geochemistry, Geophysics, Geosystems 12 (6): Q0AA18, doi.org/10.1029/2010GC003478.
McLean, N. M., D. J. Condon, B. Schoene, and S. A. Bowring. 2015. “Evaluating Uncertainties in the Calibration of Isotopic Reference Materials and Multi-Element Isotopic Tracers (EARTHTIME Tracer Calibration Part II). Geochimica et Cosmochimica Acta 164: 481–501.
McLean, N. M., J. F. Bowring, and G. Gehrels. 2016. “Algorithms and Software for U-Pb Geochronology by LAICPMS.” Geochemistry, Geophysics, Geosystems 17 (7): 2480-2496, doi:10.1002/2015GC006097.
Miller, J. L. 2012. “Time to Reset Isotopic Clocks? Two New Studies Revise Key Parameters in Radiometric Dating.” Physics Today 65 (6): 20–22.
Min K., R. Mundil, P. R. Renne, and K. R. Ludwig. 2000. “A Test for Systematic Errors in 40Ar/39Ar Geochronology through Comparison with U-Pb Analysis of a 1.1-Ga Rhyolite.” Geochimica et Cosmochimica Acta 64 (1): 73–98.
Mundil, R., I. Metcalfe, K. R. Ludwig, P. R. Renne, F. Oberli, and R. S. Nicoll. 2001. “Timing of the Permian-Triassic Biotic Crisis: Implications from New Zircon U/Pb Age Data (and Their Limitations).” Earth and Planetary Science Letters 187 (1–2): 131–145.
Murphy, D. T., B. S. Kamber, and K. D. Collerson. 2003. “A Refined Solution to the First Terrestrial Pb-Isotope Paradox.” Journal of Petrology 44 (1): 39–53.
Nier, A. O. 1938. “Variations in the Relative Abundances of the Isotopes of Common Lead from Various Sources.” Journal of the American Chemical Society 60 (7): 1571–1576.
Nier, A. O. 1940. “A Mass Spectrometer for Routine Isotope Abundance Measurements.” Reviews of Scientific Instruments 18: 398–411.
Nier, A. O., R. W. Thompson, and B. F. Murphey. 1941. “The Isotopic Constitution of Lead and the Measurement of Geologic Time. III.” Physical Review 60: 112–116.
Papanastassiou, D. A., and G. J. Wasserburg. 1971. “Rb-Sr Ages of Igneous Rocks from the Apollo 14 Mission and the Age of the Fra Mauro Formation.” Earth and Planetary Science Letters 12 (1): 36–48.
Paton, C., J. D. Woodhead, J. C. Hellstrom, J. M. Hergt, A. Grieg, and R. Maas. 2010. “Improved Laser Ablation U-Pb Zircon Geochronology through Robust Downhole Fractionation Correction.” Geochemistry, Geophysics, Geosystems 11 (3): Q0AA06, doi:10.1029/2009GC002618.
Paton, C., J. C. Hellstrom, B. Paul, J. D. Woodhead, and J. M. Hergt. 2011. “Iolite: Freeware for the Visualisation and Processing of Mass Spectrometric Data.” Journal of Analytical Atomic Spectrometry 26 (12): 2508–2518.
Patterson, C. 1956. “Age of Meteorites and the Earth.” Geochimica et Cosmochimica Acta 10 (4): 230–237.
Petrus, J. A., and B. S. Kamber. 2012. “VizualAge: A Novel Approach to Laser Ablation ICP-MS U-Pb Geochronology Data Reduction.” Geostandards and Geoanalytical Research 36 (3): 247–270.
Phipps Morgan, J., and W. J. Morgan. 1999. “Two-Stage Melting and the Geochemical Evolution of the Mantle: A Recipe for Mantle Plum-Pudding.” Earth and Planetary Science Letters 170 (3): 215–239.
Potts, P. J. 2012. “Glossary of Analytical and Metrological Terms from the International Vocabulary of Metrology (2008).” Geostandards and Geoanalytical Research 36 (3): 231–246.
Purdom, G., and A. A. Snelling. 2013. “Survey of Microbial Composition and Mechanisms of Living Stromatolites of the Bahamas and Australia: Developing Criteria to Determine the Biogenicity of Fossil Stromatolites.” In Proceedings of the Seventh International Conference on Creationism. Edited by M. Horstemeyer, 1–32. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Renne, P. R., D. B. Karner, and K. R. Ludwig. 1998. “Absolute Ages Aren’t Exactly.” Science 282 (5395): 1840–1841.
Richards, J. R. 1971. “Major Lead Orebodies—Mantle Origin?” Economic Geology 66 (3): 425–434.
Rollinson, H. R. 1993. Using Geochemical Data: Evaluation, Presentation, Interpretation. Essex, England: Longman.
Rudnick, R. L., and S. Gao. 2005 “Composition of the Continental Crust.” In The Crust. Edited by R. L. Rudnick. Treatise on Geochemistry, Vol. 3. Edited by H. D. Holland and K. K. Turekian, 1–64. Amsterdam, The Netherlands: Elsevier.
Rutherford, E. 1906. Radioactive Transformations. New York: Charles Scribner’s Sons.
Schaltegger, U., A. K. Schmitt, and M. S. A. Horstwood. 2015. “U-Th-Pb Zircon Geochronology by ID-TIMS, SIMS, and Laser Ablation ICP-MS: Recipes, Interpretations, and Opportunities.” Chemical Geology 402: 89–110.
Schärer, U. 1984. “The Effect of Initial 230Th Disequilibrium on Young U-Pb Ages: The Makalu Case, Himalaya.” Earth and Planetary Science Letters 67: 191–204.
Schmitz, M. D., and S. A. Bowring. 2001. “U-Pb Zircon and Titanite Systematics of the Fish Canyon Tuff: An Assessment of High-Precision U-Pb Geochronology and Its Application to Young Volcanic Rocks.” Geochimica et Cosmochimica Acta 65 (15): 2571–2587.
Schmitz, M. D., and B. Schoene. 2007. “Derivation of Isotope Ratios, Errors, and Error Correlations for U-Pb Geochronology Using 205Pb-235U-(233U)-Spiked Isotope Dilution Thermal Ionization Mass Spectrometric Data.” Geochemistry, Geophysics, Geosystems 8 (8): Q08006, doi:10.1029/2006GC001492.
Schmitz, M. D. 2012. “Radiogenic Isotope Geochronology.” In The Geologic Time Scale 2012. Edited by F. M. Gradstein, J. G. Ogg, M. D. Schmitz, and G. M. Ogg. Vol. 1, chapter 6, 115–126. Amsterdam, The Netherlands: Elsevier.
Schoene, B., J. L. Crowley, D. J. Condon, M. D. Schmitz, and S. A. Bowring. 2006. “Reassessing the Uranium Decay Constants for Geochronology Using ID-TIMS U–Pb Data.” Geochimica et Cosmochimica Acta 70 (2): 426–445.
Schoene, B., D. J. Condon, L. Morgan, and N. M. McLean. 2013. “Precision and Accuracy in Geochronology.” Elements 9 (1): 19–24.
Schön, R., G. Winkler, and W. Kutschera. 2004. “A Critical Review of Experimental Data for the Half-Lives of the Uranium Isotopes 238U and 235U.” Applied Radiation and Isotopes 60 (2–4): 263–273.
Snelling, A. A. 2000. “Geochemical Processes in the Mantle and Crust.” In Radioisotopes and the Age of the Earth: A Young-Earth Creationist Research Initiative. Edited by L. Vardiman, A. A. Snelling, and E. F. Chaffin, 123–304. El Cajon, California: Institute for Creation Research, and St. Joseph, Missouri: Creation Research Society. http://www.icr.org/rate/.
Snelling, A. A. 2005a. “Radiohalos in Granites: Evidence for Accelerated Nuclear Decay.” In Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative. Edited by L. Vardiman, A. A. Snelling, and E. F. Chaffin, 101–207. El Cajon, California: Institute for Creation Research, and Chino Valley, Arizona: Creation Research Society. http://www.icr.org/article/radiohalos-granites-evidence-for-accelerated/.
Snelling, A. A. 2005b. “Fission Tracks in Zircons: Evidence for Abundant Nuclear Decay.” In Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative. Edited by L. Vardiman, A. A. Snelling, and E. F. Chaffin, 209–324. El Cajon, California: Institute for Creation Research, and Chino Valley, Arizona: Creation Research Society. http://www.icr.org/article/fission-tracks-zircons-evidence-for/.
Snelling, A. A. 2009. Earth’s Catastrophic Past: Geology, Creation and the Flood. Green Forest, Arkansas: Master Books; and Petersburg, Kentucky: Answers in Genesis.
Snelling, A. A. 2014a. “Determination of the Radioisotope Decay Constants and Half-Lives: Rubidium-87 (87Rb).” Answers Research Journal 7: 311–322. https://answersingenesis.org/geology/radiometric-dating/determination-radioisotope-decay-constants-and-half-lives-rubidium-87-87Rb/.
Snelling, A. A. 2014b. “Determination of the Radioisotope Decay Constants and Half-Lives: Lutetium-176 (176Lu).” Answers Research Journal 7: 483–497. https://answersingenesis.org/geology/radiometric-dating/determination-radioisotope-decay-constants-and-half-lives-lutetium-176/.
Snelling, A. A. 2015a. “Determination of the Radioisotope Decay Constants and Half-Lives: Rhenium-187 (187Re).” Answers Research Journal 8: 93–111. https://answersingenesis.org/geology/radiometric-dating/determination-radioisotope-decay-constants-and-half-lives-rhenium-187/.
Snelling, A. A. 2015b. “Determination of the Radioisotope Decay Constants and Half-Lives: Samarium-147 (147Sm).” Answers Research Journal 8: 305–321. https://answersingenesis.org/geology/radiometric-dating/determination-radioisotope-decay-constants-and-half-lives-samarium-147/.
Snelling, A. A. 2016. “Determination of the Radioisotope Decay Constants and Half-Lives: Potassium-40 (40K).” Answers Research Journal 9: 171–196. https://answersingenesis.org/ geology/radiometric-dating/determination-radioisotopedecay-constants-half-lives-potassium-40/.
Snelling, A. A. 2017. “Determination of the Decay Constants and Half-Lives of Uranium-238 (238U) and Uranium-235 (235U), and the Implications for U-Pb and Pb-Pb Radioisotope Dating Methodologies.” Answers Research Journal 10: 1–38. https://answersingenesis.org/geology/radiometric-dating/determination-decay-constants-half-lives-uranium/.
Stacey, J. S., and J. D. Kramers. 1975. “Approximation of Terrestrial Lead Isotope Evolution by a Two-Stage Model.” Earth and Planetary Science Letters 26 (2): 207–221.
Stanton, R. L., and R. D. Russell. 1959. “Anomalous Leads and the Emplacement of Lead Sulfide Ores.” Economic Geology 54 (4): 588–607.
Steiger, R. H., and E. Jäger. 1977. “Subcommission on Geochronology: Convention on the Use of Decay Constants in Geo- and Cosmochronology.” Earth and Planetary Science Letters 36 (3): 359–362.
Tatsumoto, M., and J. N. Rosholt. 1970. “Age of the Moon: An Isotopic Study of U-Th-Pb Systematics of Lunar Samples.” Science 167 (3918): 461–463.
Tatsumoto, M., R. J. Knight, and C. J. Allègre. 1973. “Time Differences in the Formation of Meteorites as Determined from the Ratio of Lead-207 to Lead-206.” Science 180 (4092): 1279–1283.
Taylor, T. M., N. W. Jones, and S. Moorbath. 1984. “Isotopic Assessment of Relative Contributions from Crust and Mantle Sources to the Magma Genesis of Precambrian Granitoid Rocks.” Philosophical Transactions of the Royal Society of London A 310 (1514): 605–625.
Tera, F. 1983. “U-Th-Pb in Chondrites—Evidence of Elemental Mobilities and the Singularity of Primordial Pb.” Earth and Planetary Science Letters 63 (2): 147–166.
Tera, F. 2003. “A Lead Isotope Method for the Accurate Dating of Disturbed Geologic Systems: Numerical Demonstrations, Some Applications and Implications.” Geochimica et Cosmochimica Acta 67 (19): 3687–3715.
Tera, F. 2006. “Lead Isotope Planetary Profiling (LIPP): Summation-Depiction of Earth’s Many Reservoirs.” Chemical Geology 233 (1–2): 1–45.
Tera, F. 2017a. “Theory of a Methodology for Initial Lead Determination, and a Procedure to Resolve it to Some of its Stages.” Chemical Geology 450: 1–17.
Tera, F. 2017b. “Determination of Initial Leads of Four Terrestrial Terrains, Applying the Tulip Methodology.” Chemical Geology 450: 18–30.
Tera, F., and G. J. Wasserburg. 1972. “U-Th-Pb Systematics in Three Apollo 14 Basalts and the Problem of Initial Pb in Lunar Rocks.” Earth and Planetary Science Letters 14 (3): 281–304.
Tera, F., and G. J. Wasserburg. 1973. “A Response to a Comment on U-Pb Systematics in Lunar Basalts.” Earth and Planetary Science Letters 19 (2): 213–217.
Tera, F., and G. J. Wasserburg. 1974. “U-Th-Pb Systematics on Lunar Rocks and Inferences about Lunar Evolution and the Age of the Moon.” Proceedings of the Fifth Lunar Conference, Geochimica et Cosmochimica Acta 2 (Supplement 5): 1571–1599.
Thielemann, F.-K., A. Arcones, R. Käppeli, M. Liebendörfer, T. Rauscher, C. Winteler, C. Fröhlich, et al. 2011. “What are the Astrophysical Sites for the r-Process and the Production of Heavy Elements?” Progress in Particle and Nuclear Physics 66 (2): 346–353.
Thomson, J. J. 1911. “A New Method of Chemical Analysis.” Nature 86 (2170): 466–469.
Tissot, F. L. H., and N. Dauphas. 2015. “Uranium Isotopic Compositions of the Crust and Ocean: Age Corrections, U Budget and Global Extent of Modern Anoxia.” Geochimica et Cosmochimica Acta 167: 113–143.
Turner, G., J. C. Huneke, F. A. Podosek, and G. J. Wasserburg. 1971. “40Ar-39Ar Ages and Cosmic-Ray Exposure Ages of Apollo 14 Samples.” Earth and Planetary Science Letters 12 (1): 19–35.
van Keken, P. E., E. H. Hauri, and C. J. Ballentine. 2002. “Mantle Mixing: The Generation, Preservation, and Destruction of Chemical Heterogeneity.” Annual Review of Earth and Planetary Sciences 30: 493–525.
Vardiman, L., A. A. Snelling, and E. F. Chaffin, eds. 2000. Radioisotopes and the Age of the Earth: A Young-Earth Creationist Research Initiative. El Cajon, California: Institute for Creation Research, and St. Joseph, Missouri: Creation Research Society. http://www.icr.org/rate/.
Vardiman, L., A. A. Snelling, and E. F. Chaffin, eds. 2005. Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative. El Cajon, California: Institute for Creation Research, and Chino Valley, Arizona: Creation Research Society. http://www.icr.org/rate2/.
Villa, I. M., M. L. Bonardi, P. De Bièvre, N. E. Holden, and P. R. Renne. 2016. “IUPAC-IUGS Status Report on the Half-Lives of 238U, 235U and 234U.” Geochimica et Cosmochimica Acta 172: 387–392.
Wasserburg G. J., D. A. Papanastassiou, E. V. Nemov, and C. A. Bauman. 1969. “A Programmable Magnetic Field Mass Spectrometer with On-Line Data Processing.” Reviews of Scientific Instruments 40 (2): 288–295.
Watson, E. B., D. J. Cherniak, J. M. Hanchar, T. M. Harrison, and D. A. Wark. 1997. “The Incorporation of Pb in Zircon.” Chemical Geology 141 (1–2): 19–31.
Wetherill, G. W. 1956. “Discordant Uranium-Lead Ages.” EOS, Transactions of the American Geophysical Union 37 (3): 320–326.
Wetherill, G. W. 1963. “Discordant Uranium-Lead Ages—Pt. 2. Discordant Ages Resulting from Diffusion of Lead and Uranium.” Journal of Geophysical Research 68 (10): 2957–2965.
Wetherill, G. W. 1966. “Radioactive Decay Constants and Energies.” In Handbook of Physical Constants. Edited by S. P. Clark Jr., Geological Society of America Memoir 97, 514–519. Boulder, Colorado: Geological Society of America.
White, W. M. 2015. “Isotopes, DUPAL, LLSVPs, and Anekantavada.” Chemical Geology 419: 10–28.
Wiedenbeck, M., P. Allé, F. Corfu, W. L. Griffin, M. Meier, F. Oberli, A. von Quadt, J. C. Roddick, and W. Spiegel. 1995. “Three Natural Zircon Standards for U-Th-Pb, Lu-Hf, Trace Element and REE Analyses.” Geostandards Newsletter 19: 1–23.
Williams, I. S. 1998. “U-Th-Pb Geochronology by Ion Microprobe.” In Applications of Microanalytical Techniques to Understanding Mineralizing Processes. Edited by M. A. McKibben, W. C. Shanks III, and W. I. Ridley. Reviews in Economic Geology 7: 1–35. San Diego, California: Economic Geology Publishing Company.
Zack, T., D. F. Stockli, G. L Luvizotto, M. G. Barth, E. Belousova, M. R. Wolfe, and R. W. Hinton. 2011. “In Situ U-Pb Rutile Dating by LA-ICP-MS: 208Pb Correction and Prospects for Geological Applications.” Contributions to Mineralogy and Petrology 162 (3): 515–530.
Zartman, R. E., and S. M. Haines. 1988. “The Plumbotectonic Model for Pb Isotopic Systematics Among Major Terrestrial Reservoirs—A Case for Bi-directional Transport.” Geochimica et Cosmochimica Acta 52 (6): 1327–1339.
Zindler, A., and S. R. Hart. 1986. “Chemical Geodynamics.” Annual Review of Earth and Planetary Sciences 14: 493–571.












