The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
This third installment is a continuation of my response to a recent critical paper (Jeanson 2013) of a series of Acts & Facts articles published by the Institute for Creation Research (ICR). The series contrasted a programmed design innate to organisms that enables them to fill environmental niches versus natural selection. For brevity, I refer to the critical paper as “the critique” and the Acts & Facts articles as the “series.” Part 3 will try to meet the critique’s request (at least in part) to provide additional biological evidence supporting the series’ claim that organisms self-adjust to external conditions by expressing traits that succeed (or fail) to solve the challenges posed by dynamic external conditions. Representative examples highlight multiple innate mechanisms across diverse taxa demonstrating that successful trait designs determine whether an organism fills a new environment, abides in place, or, post-Fall, possibly dies. A linkage between challenges, traits, and their underlying information-based mechanism is shown that it can be objectively verified. In the selectionist’s worldview which is heavily influenced by naturalism, credit for the success or failure of an organism’s traits to solve environmental challenges is misacribed to the environment. Traits are said to be “due to” the molding of organisms as they are driven through time by “selective pressures.” Primary causality is deflected to the environment when that objective success of an organism’s trait is mislabled “selected for” (or its failure misleadingly called “selected against”) in an undetectable/mystical selection event as environments exercise their agency. The mechanisms detailed below show, contrary to selectionist scenarios, that traits do not “arise” due to so-called “selective pressures” driving organismal adaptation. But give reason to believe that organism’s innate systems a) already possess information or capacity to solve environmental challenges (including some that have not even appeared on earth yet,) and b) control mechanisms to potentially express novel genetic sequence or control genetic information in real time. Other studies elucidate interconnected detection, control, and actuator systems effecting how various environmental exposures are detected by system elements, which for example, can then initiate epigenetic transgenerational inheritance of phenotypic variation. Genomic features are identified associated with sperm epimodifications that could illuminate how these sites are utilized in rapid transgenerational programming. This part demonstrates that all of these outputs are only representative of a broad capacity intrinsic to an organism’s systems and, therefore, are not “hidden designs” whose expression is “mediated” by environments exercising agency (via “mediating” actions that are yet undetectable). Finally, recognition is due to several other creationists (e.g., Lightner, Borger) who clearly have been very progressive in encapsulating systems such as those directing specific mutations and Variation Inducing Genetic Elements (VIGEs) designed to control self-adjustments that strongly suggest organisms express incredible engineered adaptability.
Keywords: epigenetic, regulatory network, systems, engineered adaptability, design analysis, adaptation, natural selection, selectionist, self-adjusting phenotype
Design-Based Insights to Organism-Environment “Interactions”
This part presents biological examples of how the seven design principles explained in Part 2 are expressed in living things. These examples also support the series’ claim that organisms self-adjust to external conditions by expressing traits that succeed (or fail) to solve the challenges posed by dynamic external conditions which are the true, primary cause underlying whether an organism fills a new environment, abides in place, or, post-Fall, possibly dies.
How do design-based systems analysis approach the fact that entities do adaptively “interact” with their environments?
First, in terms of causation, entities do not really “act” on each other. Readers of all three parts of this paper—merely an exposure to the reader—may change their thinking from an environment-driven to an organism-driven explanation of self-adjustment. But this paper did not cause the change of thinking in the reader. The reader self-adjusts himself. This paper is not even a so-called “passive cause.” Exposures cannot skip past the boundaries of any entity (i.e., “inter”) and literally change (i.e., “act”) its wholesale processes in some manner independent of its systems (Cabej 2011, pp. 2–3).
Second, design analysis recognizes levels of design higher than that within individual entities. Two entities, in the normal outworking of their individual functions, may themselves be designed to work together such as a radio transmitter and a radio as parts of an overall communications system. These higher levels of design are easily explained by design analysis, but evolutionists struggle with these explanations—particularly between parts or organisms which seem to be codependent on each other. Evolutionists will generally appeal to either mystical explanations or skip large sections of an organism’s innate design.
A recent example of the latter is how evolutionists claimed that they had a documented case of dependent species arising by natural processes in the fly Drosophila pachea on its single host, the senita cactus. But what they documented were mutations (the cause of mutations was undetermined) which presumably destroyed the function of a gene. This gene’s products were integrated into a complex biochemical pathway which previously allowed the fly to live on multiple plant sources of sterol nutrients (already including the senita cactus). The fly is now restricted to a type of sterol found in the senita cactus that the fly can process without the broken genes’ product—which it could do all along (Lang et al. 2012, p. 1661). D. pachea now fills an ecological niche which its traits define as containing senita cacti, but nothing new arose in either the fly or the plants.
The naturalistic environmentalist approach to the origins of the connections between an organism and its environment excludes the explanation of intelligent design from the start. Thus, the environment is responsible for both building the capacity of developing embryos to adapt to changing conditions, and then directing the changes themselves as Gilbert summarizes, “It [ecological developmental biology] focuses on how animals have evolved to integrate signals from the environment into their normal developmental trajectories” (Gilbert and Epel 2009, p. 9). In contrast, based on design analysis, embryos would be seen as having been programmed with innate designed mechanisms to detect external conditions during development (or from information passed on from a parent’s detection) which self-adjust their normal developmental trajectories which results in physical traits best suited to fill a changed environment.
In contrast, this evidence indicates that organisms’ innate systems already possess information or capacity to solve environmental challenges (including some that have not even appeared on earth yet,) which do not support selectionist scenarios, and that traits “arise” due to so-called “selective pressures” driving organismal adaptation. For instance, tomcod fish already possessed genes to detoxify man-made polychlorinated biphenyls (PCBs) pollutants (Thomas 2011) and other organisms are successfully filling the highly radioactive niche around the Chernobyl accident (Thomas 2014) (niches perhaps not in the original creation) via traits that control both radiation exposure to DNA and enhance its repair mechanisms.
Designed Self-Adjusting Mechanisms in Living Things
The similarity between self-adjusting man-made entities and living entities is most easily seen in how organisms maintain homeostasis through physiological self-adjustments. Organisms have sensors which detect changes in external or internal conditions and encode the data which is input to a logic center. The data is decoded and compared to an information bank specifying a range of “normal” in the center which then encodes an instructional output message sent to some type of target part. This part decodes the message and produces selected responses to try to maintain homeostasis in the face of changed conditions.
Yet, can it be said that what is true of physiologic self-adjustment is also true of adjustments which can be made between parent and offspring or in a whole population of organisms over multiple generations? As Kirschner and Gerhart (2005) noted previously, the road to long-term phenotypic adaptation to different environments is paved with physiological adaptability—since they often entail the same cellular mechanisms. These mechanisms also work to self-adjust offspring to changing internal and external conditions primarily, though not exclusively, during embryonic development (this is true of both plants and animals but plants are able to express a larger range of real-time adjustments). The observation of rapid intergenerational changes happening with varying external conditions goes back over 100 years.
Resilient Organisms: Distinguished by Plastic, Self-Adjusting Phenotypes
Developmental biologist Scott Gilbert reports on an important paper published out of Germany in 1909 in which genetically identical populations of the water flea, Daphnia, produced different phenotypes in offspring when facing the challenges of different seasons or different predators. With considerable thought based on these observations, the paper made an early argument that the genetic information inherited by Daphnia offspring conferred not a static phenotype but the potential to generate a large number of very small variations in phenotype which the paper labeled a “reaction norm” or “norm of reaction” (Gilbert and Epel 2009, p. 8).
Information-Based Internal Systems Control Expression of Plastic Phenotypes
Sophisticated man-made, self-adjusting devices would integrate exquisitely sensitive detectors capable of sending non-stop data to a powerful, high-speed computer which directs precisely adjustable output components. Devices like these achieve essentially an infinite number of self-adjusted states within a fixed range. This type of performance simulates the way a reaction norm functions within individuals of a population of organisms. The genome specifies a continuous range of potential phenotypes bounded by upper and lower limits of action. As each embryo within a population develops, they collect data about conditions of their individual environment (Waterland, Travisano, and Tahiliani 2007, p. 3380) which is utilized by their internal developmental program to direct the formation of their traits.
Embryological development is highly regulated. Good information starts with collecting large quantities of high quality data—which begins with a sensor.
Consider the analysis by a developmental biologist of data-transmitting potential for just one receptor for a growth factor (PDGF) involved in development of different body plans. “The typical receptor has many different potential autophosphorylation sites (in the case of the PDGF receptor at least ten), and it is highly unlikely that all sites can be phosphorylated at the same time . . . . If each of the 10 sites can be phosphorylated or dephosphorylated independently of the others, the total number of potential phosphorylation states per receptor will be 210 (1,024). But because receptors must dimerize in order to activate, each activated receptor dimer has a much larger number of potential states— in this case, more than 500,000 different unique combinations of phosphorylation states . . . The state of phosphorylation is critically important because it is these very phosphorylation sites that serve to transmit downstream signals from the activated receptor. They do so by binding to cytosolic effector proteins . . . There are more than 100 of these cytosolic effector proteins that can bind to the receptor . . . Effector binding leads to a tremendous increase in the number of potential states for the receptor. Even if we oversimplify and assume that each phosphorylated site can bind to only one effector . . . the total potential number of states for each receptor monomer increases to 310 (around 60,000) and for the receptor dimer to almost 2 billion! This does not even take into consideration the possibilities that any bound effector may or may not be phosphorylated by the receptor, or be simultaneously bound to yet another effector. Clearly, the theoretical number of possible states is virtually infinite, . . .” (Mayer, Blinov, and Loew 2009, p. 81.2).
The potential data of a very complicated sensor would be wasted unless it was coupled to a very sophisticated regulatory network. Complex regulatory networks control cells during embryologic development and thereafter. Networks are intracellular logic paths with information to specify performance which means that they operate like volition—since “specifying” means to consciously select or exclude.
It also looks like it is front-loaded to optimize the organism for the environment it will live in. Management of genes may be controlled by “promoter” DNA. Control of the promoter is achieved by other products (either DNA or proteins) called “regulators” that can activate or suppress promoters. Often, multiple regulators control promoters, and they may control each other via internal logic strategies consisting of “AND gates,” “OR gates,” “feed forward (or back) loops,” “if-then gates,” or “toggle switches” (Alon 2007, p. 459). Analysis of the organization of human regulatory information led researchers to conclude that, “A number of clear design principles emerge from it” (Gerstein et al. 2012, p. 98). All of these may respond to concentrations of regulators or protein products. Regulators are generally activated via the neuroendocrine or central nervous systems which transduce signals from receptors or sensors (often embedded in the cell membrane) which detect different external conditions (Cabej 2005, p. 51; Nijhout 2003, p. 9).
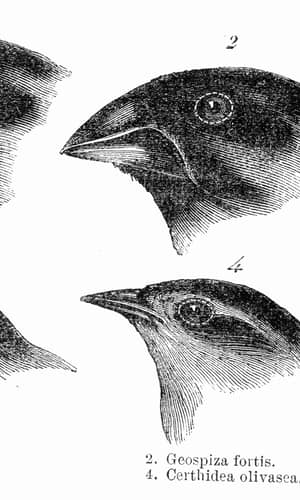
Clearly, networks yield abundant combinations with extensive results ranging from protein synthesis to forming all different types of cells. Broader regulations direct the shapes of diverse organisms from larger (often similar) portions of DNA (Carroll 2005, p. 65). And similar networks exist in humans to bacteria which control traits via regulation of the expression of genes. Complex developmental programs utilize numerous means (including controlled expression of mutation) to regulate genes expression in different locations, times, amounts, and types. The result is a bewildering range of trait variation which seems to enable the organism to fit and fill ever-changing ecological niches which is classically exemplified in the different beak types of Darwin’s finches. A bird’s diet (Bowman 1961, p. 15) is highly dependent on its beak’s three dimensional shapes which are a function of numerous regulated factors directing its craniofacial development as an embryo (Abzhanov et al. 2004, p. 1463; Abzhanov et al. 2006, p. 563; Lightner 2012, p. 8).
Resilient Organisms: Distinguished by Plastic Self-Adjusting Phenotypes
An illustration of the outworking of informational functions of particular proteins or the embryo’s developmental program is as “if-then” commands which specify one of two discreet “either-or” trait outcomes called “morphs.” This type of self-adjusting plasticity is called “polyphenism” and must be found to happen in 5% or more of the population to distinguish this outcome from that which may flow from mutation-related informational changes.
Even as embryos, organisms must have exquisite detectors for sensing external conditions or have proteins that perform in different ways given different conditions or other innate mechanisms to trigger embryonic self-adjustments (Murdock and Wibbels 2006, p. 134; Shoemaker et al. 2007, p. 1059). For example, embryologic development of some species of turtle (Crain and Guillette 1998, p. 77) and crocodiles (Woodward and Murray 1993, p. 149) self-adjusts in different ground temperatures around the eggs resulting in different proportions of male-female sex ratios (up to 100% of one sex.) The gene for expression of the hormone aromatase which converts testosterone to estrodiol is tightly regulated by other gene products given different external temperatures. Aromatase binds to different receptors in the embryo which activates other genes in the system for sex-determination system. This phenomenon may allow a longer time for sexual maturation of females and possibly have nothing to do with survival in itself.
Environmentalists are forced to ascribe causality for these phenomena to the environment only after they jump over key elements in the system, as Shoemaker et al. do in their paper on sexual development in the turtle Trachemys scripta where they say, “In other vertebrates, environmental factors direct sexual development, such as in temperature-dependent sex determination (TSD) found in all crocodilians and many turtles” (emphasis added) (Shoemaker et al. 2007, p. 1055). Do environmental factors really direct sexual development or doesn’t design analysis show that for some vertebrates it’s their innate program’s specifications (e.g., if [x] temperature is detected, then develop male sex) as the true director of sexual development?
A temperature cannot directly transcribe genes in an organism. Later in the paper where they describe in more detail the function of the system and are forced to speculate on the link between temperature and gene transcription they say that in contrast to genetic sex specification, “Alternatively, an undetermined upstream factor may sense and respond to a male-producing temperature and upregulate both Sox9 and Mis [a gene and growth factor involved in sex determination]” (Shoemaker et al. 2007, p. 1059). Of course there must be something in the organism which senses temperature and sends internal signals to initiate gene transcription. Were it not for the a priori bias against design, it may be apparent that environmental factors neither direct sexual development nor determine “male-producing” temperatures. Rather, internal conditional programming specifies these temperatures, which are coupled to an indispensable sensing element to gather temperature data—which essentially is the trigger for all of the follow-on elements in the system.
In total contrast, Cabej notes how the above example, and many similar examples, shows that in the outworking of transgenerational developmental plasticity, external stimuli, per se, cannot induce expression of any gene (Cabej 2011, p. 4).
The programming of some butterfly species adjusts wing pigmentation depending on the larva’s exposure to seasonal photoperiods and external temperature (Nijhout 1999, p. 181). Pigmentation along with the size and number of “eyespots” on the wings are regulated by the Distal-less gene. Control of this gene’s expression is by the hormone ecdysone which is variably released by endocrine signals sent from a temperature sensing element in the larva (Beldade, Brakefield, and Long 2002, p. 316).
Sensor Initiated Systems Adapt Organisms to Fill New Niches
Researchers familiar only with older studies on physiologic adaptations may not readily see parallel and dual uses of systems used for both physiologic and genetic adaptation. Still believing that all genetic adaptation is poorly understood, they would question what “sensors” did the felid “kind” ancestor have to grow a lion mane, or “detection mechanism” did the equid “kind” ancestor have to grow zebra stripes or what sensors did the canid “kind” ancestor have to grow a bushy wolf tail? If it is given (which is unlikely) that all of those traits are adaptive or are not “free riders” tagging along with truly adaptive traits, could current research also show: 1) that sensors utilized in physiological adaptations could also be elements of systems that manipulate larger changes in embryological development; 2) that distinct sensors exist utilized in systems for large morphological change(s); or 3) that processes exist to assimilate genetic code for physiologic adaptations or retain epigenetic changes for multiple generations? The answer is yes, and this is why, as Kirschner and Gerhart noted previously, the road to genetic adaptation is paved with physiological adaptability—since they often entail the same cellular mechanisms as is seen in the following examples.
Insect
The migratory locust Schistocerca gregaria can exist in two very different forms. One form looks and behaves much like an ordinary grasshopper in uniform color and solitary behavior, while the other form is brightly colored, has long wings, and socializes in large groups as they migrate to new territory (Thomas 2009). In what seems like a programmed mechanism to adjust the density of grasshopper populations, locust nymphs have elaborate innate mechanisms to monitor the counts of which mechanosensory hairs on their hind legs are touched by other hoppers per a given unit of time (coupled with olfactory and visual data) (Rogers et al. 2003, p. 4000). These sensors send signals mediated by an increase in serotonin in the thoracic ganglia which can lead to a rapid molt in which non-social forms morph into the migratory social form. This self-adjustment can happen rapidly as the researchers found, “Repeatedly touching as little as one quarter of the anterior (outer) surface area of a hind femur produced full behavioural gregarization within 4 h” (ibid, p. 3991). Interestingly, the Australian plague locust, Chortoicetes terminifera, also molts to a migratory gregarious form but is programmed to monitor, “tactile stimulation of the antennae, with no evidence for an effect of visual and olfactory stimulation or tactile stimulation of the hind legs” (Cullen et al. 2010, p. 937).
Fish
Another example is how coral reef fish migrate to new reef territories but also face the challenge of finding a mate in the new areas in order to reproduce. Multiple studies demonstrate that innate programming enables different varieties of these fish to rapidly self-adjust to the complimentary sex after detecting, generally an older fish, in new territories. The fish visually detect a potential mate and internal signals are sent which activate one of several preprogrammed routes of the fish’s neuroendocrine system to hormonally control its gonad maturation (Godwin, Luckenbach, and Borski 2003, p. 40). One definitive study on the obligate coral-dwelling goby, Gobiodon erythrospilus, indicated considerable plasticity in gonad maturation and sex determination. Flexibility in the timing of maturation allows single immature juveniles to maintain a labile sex determination which enables a juvenile to form a breeding pair with any adult encountered (Hobbs, Munday, and Jones 2004, p. 2113).
In some species the adult forms maintain sexual plasticity as in the blue-headed wrasse, Thalassoma bifasciatum, in which the ovaries of a large resident female will regress and testes grow within a single day if a territorial phase male is lost (Warner and Swearer 1991, p. 204).
In regard to the central question of what is the underlying cause of sex changes in fish, naturalistic environmentalists will ascribe causality to the environment at the expense of the organism by skipping over some of its important anatomical and physiologic features. For example, evolutionary biologist Scott Gilbert asserts, “In both Gobys and the blue-headed wrasse, the shift in sex is mediated by hormonal changes caused by the environmental conditions” (Gilbert and Epel 2009, p. 32, emphasis added).
In a misleading attribution of information to the environment and away from organisms, a lead researcher said, “Because of the central role of aromatase in the biosynthesis of estrogens, it will be a focus in consideration of mechanisms by which environmental information leads to sex determination responses. More generally, our understanding of vertebrate sexual function indicates the HPG [hypothalamo-pituitary-gonadal] axis plays the key role in transducing environmental information into gonadal determination, differentiation, and maturation events” (Godwin, Luckenbach, and Borski 2003, p. 40 emphasis added).
The researchers’ papers only documented the presence or absence of mature fish, juvenile fish, sex change, etc. but not that the environment possessed any information, components, or means to qualify as a “mechanism” determining or controlling sex change. All mechanisms described in the research including the fish’s ability to gather data on the environment and transduce it to information were innate to the fish. Individual fish self-adjust sex in the context of innate programming that specifies what external conditions will be a stimuli for its self-adjusting systems, which enables them to fit into varying social settings. These systems seem purposively designed to make adjustments at both the level of individual fish and the level of the larger social setting.
Amphibian
Amphibian resiliency is exhibited in extreme developmental plasticity in variable timing of metamorphic changes. The tadpoles of several species of toad residing in arid environments continuously monitor water availability in their breeding ponds. They modulate their metamorphic changes toward adult toad proportional to the drying of their ponds (Newman 1992, p. 671). The western spadefoot toad, Scaphiopus hammondii, is one model species. It appears that the tadpoles monitor primarily the water level and swimming area of their ponds (other pond condition variables have been ruled out). Multiple sensors initiate the tadpole’s response as one leading researcher describes, “It is possible that tadpoles perceive, through the use of the visual system, their proximity to the water’s surface and activate neuroendocrine centers that control metamorphosis” (Denver, Mirhadi, and Phillips 1998, p. 1870). When they determine that pond loss is imminent, some tadpoles in a pond will grow to enormous size and switch from being omnivores to carnivores. As they rapidly mature into a smaller form of the adult toad they consume remaining tadpoles and then quickly migrate to safety under the desert sand. Interestingly, in somewhat different environmental conditions another species of Scaphiopus (which also self-adjusts rapidly as their habitat desiccates) a few tadpoles will develop into smaller and leaner tadpoles (Newman and Dunham 1994, p. 377).
The naturalistic environmentalist, consistent with their Designer-denying, Environment-exalting worldview, errs in ascribing causality. They fail to see, that just like man-made self-adjusting things, tadpoles are stand-alone entities from their environment. They are driving their own metamorphosis bus via their components and innate programming which: define what environmental condition is a cue, detect those conditions, trigger neuroendocrine mediators, establish an action threshold, and initiate and complete a cascade of suitable adjustments.
In contrast, two environmentalists bluntly assert, “The toads are called out of hibernation by the thunder that accompanies the first spring storms in Arizona’s Sonoran Desert . . . The timing of their [Scaphiopus’] metamorphosis is controlled by the pond” (Gilbert and Epel 2009, pp. 57–58, emphasis added). Curiously, other naturalists who detailed the tadpole’s stunning capabilities still cling to some environmental causality and say, “Spadefoot toad tadpoles are a valuable model system for explaining both the proximate mechanisms (environmental cues and physiological responses) and the ultimate causes for adaptive phenotypic plasticity in amphibian metamorphosis” (Denver, Mirhadi, and Phillips 1998, p. 1859, emphasis added). The researcher’s papers only documented ponds existing in various states or water level, temperature, salinity, etc. but not possessing any information, components, or means to qualify as a “mechanism” causing metamorphosis. False mechanistic assertions of causality steal credit from the toad and the toad’s Designer.
Design-based solution-to-problem analysis is a quantifiable approach to predator-prey research, “If different species of enemy can be thought of as representing distinct environments for their victims, . . .” (Van Buskirk 2001, p. 482) then predators are only another type of environmental exposure/challenge which an organism’s traits must fit.
It should be noted that researchers of so-called “predator-induced morphological defenses” may be wrong in their explanations in at least two areas. They are first incorrect in labeling any of the defenses “predator-induced” in the sense that predators cause any morphological changes in any organisms. Barring magic, predators cannot directly cause induction of any genes or physiological changes inside of another organism. Predators must also be detected. Detection and physiological adjustments are due to systems innate to organisms as a discrete entity. Credit diversion from organisms to the environment is an expression of the naturalistic worldview. Second, there is research that some morphologic changes attributed to the presence of predators may not be directly correlated to the predator, but the presence of confounding factors suggest, “the lack of separation of correlation from causation calls for future research investigating the underlying mechanisms; and this call is well-supported by our review” (Bourdeau and Johansson 2012, p. 1181). An example of research which appears to have good support is with the tadpoles of the tree frog Hyla chrysoscelis. In experiments where these tadpoles develop in tanks, if a mosquito net cage containing larvae of a tadpole predator, the dragonfly Aeshna or Anax, is placed in the tank, those tadpoles rapidly self-adjust their development. “Tadpoles were probably unable to see or sense movements from predators within the cages,” but they have exquisite capability to, “detect waterborne chemical” substances “produced by predators” of the dragonfly larvae in the water (McCollum and Leimberger 1997, p. 616). Tadpoles consistently detected the predator. This piece of data is a reliable conditional input to the outworking of its developmental plan directing these tadpoles to form thick muscular bright red tails. More of these tadpoles escaped future predation better than tadpoles isolated from predator exposure during development (McCollum and Van Buskirk 1996, p. 583; Van Buskirk 2001, p. 483).
Another investigation researched the developmental changes in various combinations between two competitors of larval wood frogs (Rana sylvatica) and leopard frogs (R. pipiens) and two different predators of larval dragonflies (Anax spp.) or caged mudminnows (Umbra limi). The investigator, Rick Relyea, found that when competitors (without predators) were assessed during development, wood frog tadpoles increased their mouth width by 10% and their tail length by 3%, while leopard frog tadpoles increased their mouth width by 5% and did not change their tail length. However, when the caged presence of either predator to the tank was detected by both tadpoles the mouth width, and tail length of both prey species of tadpoles self-adjusted to precompetition levels without affecting the population density of either prey (Relyea 2000, pp. 2278, 2281, 2287). Additional research is expected to discover more self-adjustable traits and a combination of these traits likely work together to solve predator problems which are not explained by swimming ability alone. (Van Buskirk and McCollum 2000, p. 2157) Indeed, the systems enabling developmental change in tail morphology probably have existed since creation and had nothing originally to do with survival.
Systems Can Facilitate a Rapid, Heritable Variation for Multiple Generations
The important question is whether the plastic traits which were self-adjusted in one generation can be passed on to offspring in the next. The answer is yes. For instance, in regard to the thick muscular bright red tails of tadpoles exposed to predators, “The heritability of tail depth was high with predators (0.7) but quite low without predators (0)” (Relyea 2005, p. 863). The conclusion of this researcher in this large, well-designed study of heritability of traits of parents exposed to predators was, “that predator-induced traits can frequently be heritable, although the magnitude of heritability can be wide ranging across environments. Moreover, the plasticity of these defenses also can be heritable, . . .” (Relyea 2005, p. 864).
Other studies of the heritability of self-adjustable traits given predator exposures have been documented in plants including genetic variation (Jimson, Datura stramonium, and primrose, Oenothera biennis) (Agrawal et al. 2012, p. 115; Fornoni, Valverde, and Núñez-Farfán 2003, p. 1049) as well as estimates of narrow-sense heritability (wild radish, Raphanus Raphanistrum) (Agrawal et al. 2002, p. 2212). Australian researchers were the first to demonstrate additive genetic control within populations of offspring of compounds toxic to insect attackers when parent detects biochemicals of the attacker. They used marker-based methods and found significant narrow-sense heritability of the foliar defense compounds in a natural Eucalyptus melliodora population (Andrew et al. 2005, p. 1994).
Innate systems have elaborate logic-based networks which regulate the expression of genes. Multiple avenues of genetic control of genes have been documented (which may constitute the bulk of genetic function) which involve, “many feed-forward loops, which could be used to filter fluctuations in input stimuli” (Gerstein et al. 2012, p. 99). Mechanisms exist (both too many and too varied to detail here) for the modifiers of these genes to become fixed within a population so that one phenotype can become dominant from generation to generation (Ruden et al 2003, p. 309).
An intriguing system component is a “chaperone” molecule which helps fold proteins into a functional conformation labeled heat shock protein 90 or Hsp90. The concentration of this molecule can be proportional to stressful exposures encountered by multicellular organism and its concentration directly affects the expression of significant traits such that, “HSP90 modulation can both reveal and conceal the phenotypic effects of natural variation” (Sangster et al 2008, p. 2967).
This molecule was recently implicated as a major factor in the significant morphological variations such as loss of eyes seen blind cavefish, Astyanax mexicanus (Rohner et al 2013, p. 1375). Investigators asked, “A critical question, however, is whether a river fish finding itself suddenly trapped in a cave environment would experience a HSP90-related stress response” (ibid, p. 1374). Underpinning key sensor-system interactions, these fish can sense water conductivities which vary five-fold between water habitats in cave water compared to surface streams. Fish embryos which develop in low conductivity conditions up-regulate both Hsp90 and heat shock response genes which enabled expression of innate variability in eye size of smaller to absent. The alleles for small eye size appear to be disposed to genetic assimilation into the population. The conclusion by these researchers was that these changes “would have helped potentiate a rapid response to the cave environment” (Rohner et al. 2013, p. 1375). Prior to this discovery, an article in ICR’s series suggested an innate system-based mechanism may be the best explanation for how A. mexicanus may rapidly self-adjust to fit cave environments (Guliuzza 2012, p. 14).
Sangster and coworkers also demonstrated that Hsp90-connected polymorphisms facilitate rapid self-adjustment since they are continuously responsive to new conditions, are amenable to genetic mapping, and HSP90-dependent alleles are frequent in natural populations. Ruden and Lu documented five mechanisms of transgenerational epigenetic inheritance in Drosophila in which they suggest that all five systems might be partly explained by Hsp90 availability since it is required for formation of a common critical complex (Ruden and Lu 2008, p. 505). In an earlier landmark study using Arabidopsis, Rutherford and his coworkers showed that inactivation of Hsp90 exposed previously hidden phenotypic variation which could eventually be assimilated into the population (Rutherford and Lindquist 1998, p. 342).
Long-term spread of traits throughout populations must be explained. Selection-based scenarios explain this observation by appeals to a cycle of mystical “selection” events continuously performed by environments which select favored organisms (Purdom 2006, p. 276) yielding survivors claimed to have undergone the cryptic process of “positive selection.” In contrast, design analysis shows that as long as an organism’s systems continue to detect an environmental condition(s) they would likely continue to produce offspring with phenotypes suitable to fit those condition(s)—a system-based explanation devoid of appeals to any mystical “positive selection.” Exposure to the same environmental condition may be true for all members of a regional population. Numerous individuals with different genetic/epigenetic backgrounds (unlike a rare mutation that could appear in a single genetic background) could increase the likelihood that some backgrounds would stabilize the trait in the population.
Resilient systems regulating variable expression of DNA (epigenetic systems) facilitate limited multi-generational self-adjustments in offspring that can fill changed environments
Organisms need to self-adjust to environments which may notably change within a single generation. Accumulation of mutations within the germ line seems far too slow to be a plausible means for organisms to express traits that could solve these challenges. Another system which variably marks specific nucleotides of DNA with different molecules exerts control over how the information in DNA is expressed without changing the sequence of nucleotides. This is called the “epigenetic system” and research is showing that it is very influential in the expression of plastic phenotypes and, “may play a fundamental role in adaptation to rapidly changing environmental conditions, side by side with standard genetic variation” (Pigliucci 2003, p. 34).
Though the operation of these mechanisms remain only partially understood, evidence exists that epigenetic changes are heritable in a significant way as emphasized by a couple of the foremost investigators in this arena, “The evidence presented in the previous sections shows that the transgenerational transmission of epigenetic variations through cellular inheritance and through routes that bypass the germ line is not a rarity. Therefore, if we are to understand heredity and evolution, we need to acknowledge these different types of information transfer between generations and not focus exclusively on genetic transmission” (Jablonka and Lamb 2010, p. 163). In fact, it is possible that an organism’s epigenetic inheritance systems may play the largest role in how organisms can rapidly self-adjust within one to two generations.
Some systems for epigenetic inheritance are well documented and vary significantly between different organisms (Richards 2006, p. 397). One important characteristic of these systems is that they seem to be an almost real-time bridge between an organism’s environment and an innate mechanism to prepare its offspring for life in that environment (Chong and Whitelaw 2004, p. 692). Many types of plants have documented transgenerational inheritance (Richards 2011, p. 208), and at times expression can be intense while at other times transposons which have been silenced by an RNAi-based mechanism in one generation can be reactivated over subsequent generations (Singh, Freeling, and Lisch 2008).
Mammals have also cases of documented epigenetic inheritance facilitating offspring to fit new environments, but the expression appears to be much more controlled than in other organisms or in plants. For example, the size of mice and their coat colors differed markedly between genetically identical offspring born to different mothers who were exposed to diets containing different methyl-donor supplements during pregnancy. The researchers were able to attribute this to permanent effects on epigenetic gene regulatory mechanisms (Waterland and Jirtle 2003, p. 5300). The response of genetically identical mice offspring to a high fat diet differed if they gestated in either obese or lean mothers. An impressively thorough study showed that female offspring born to obese mothers had higher resistance to the obesity generating effects of early exposure to a high fat diet. These were due to a multitude of post-weaning changes in offspring in vital epigenetically controlled gene networks such that, “We suggest that these gene networks may be considered as new environmental sensors . . .” (Attig et al. 2013).
The above studies (and numerous others) may also support the possibility of systems controlling an early, and time-limited, plastic period in offspring in which epigenetic changes may occur resulting in further refinement of its fit to its environmental conditions. Research on epigenetic patterns in newborn rat pups is a remarkable example. One day before birth, the binding site for a transcription factor of a gene producing specific glucocorticoid receptors has no epigenetic methyl markers. One day after birth the site is marked in all pups. Systems in the pups detect a tactile exposure of attentive licking and grooming by a mother that, “initiates a cascade of intracellular events” and alter this epigenetic mark. Pups that did not experience attentive care retained the marker. Pups exposed to care lost the marker and thus produced the special glucocorticoid receptor in their brains—and these glucocorticoids better enables those rats to deal with stress throughout life (Weaver et al. 2007, p. 1756).
Predictably in researchers holding to naturalism, even after they describe incredibly innate epigenomic systems within rat pups to detect exposures and carry out all programming, they still ascribed causality to the environment, “This epigenomic programming of the exon 17 GR promoter by maternal care might serve as a model for a novel mechanism through which the social environment programs adaptation at the level of the genome” (Weaver et al. 2007, p. 1757, emphasis added). But, as other researchers have recognized (Cabej 2013, p. 199), maternal care and other environmental factors in-and-of-themselves cannot program the genome within an organism.
As in animal studies, human epidemiologic data indicates that the effects of non–DNA-altering exposures can sometimes be conveyed for several generations. Surprisingly, this epigenetic transmission seems to be more prominent through the male germ line. Several examples are illustrative. After a receptor in pregnant females incorporates the pesticide vinclozolin, epigenetic changes are propagated through the male germ line for up to four generations (Anway et al. 2005, p. 1468). A finding in humans seems consistent with obese mouse studies. Human epidemiologic data from Sweden suggest that there is an early plastic period in offspring that may be affected epigenetically—by parents or to their offspring. Children with differing nutritional exposures had sex-specific epigenetic changes that varied as to whether their paternal grandfather was an early smoker. They were transmitted through the male germ line to affect cardiovascular and diabetes mortality in at least two generations (Pembrey et al. 2006, p. 164). Similar Swedish studies showed a sex-specific association of ancestor’s food supply with multigenerational longevity (Bygren, Kaati, and Edvinsson 2001, p. 58) or cardiovascular and diabetic mortality (Kaati, Bygren, and Edvinsson 2002, p. 687).
Resilient systems must enable, in changing conditions, both the plasticity to self-adjust and robustness so as to maintain an organism’s general characteristics. Resiliency is particularly demonstrated in mammalian epigenetic systems. In mammals epigenetic inheritance is limit-controlled by two distinct reprogramming episodes in development. This ensures that predominantly “normal” genera-specific development is reiterated in each generation regardless of the environmental exposures experienced by the parent (Finnegan and Whitelaw 2008). Epigenetic information carried in sperm and egg genomes is mostly erased during preimplantation development. In early cell differentiation of the embryo, it is reset to the general pattern of its lineage so that cells of the embryo can reacquire pluripotency. New epigenetic information may be added during development, but late in fetal development after its primordial germ cells have formed another round of epigenetic erasure and resetting occurs (Reik, Dean, and Walter 2001, p. 1093). However, these will be altered during the lifetime of the organism consistent with its exposures. Why some sequences escape reprogramming and are likely involved in epigenetic inheritance is not fully understood.
Not only did our analysis of linear selection gradients allow us to determine that the induction was an extension of how morphology influences survival in noninduced tadpoles, but also the analysis of nonlinear selection suggested that there is a limit to the extent to which induced morphological changes can enhance survival. (Kraft, Franklin, and Blows 2006, p. 457)
Benefits of Design Analysis as Applied to the Above Systems
Design Analysis Brings Great Clarity to the Understanding of Adaptation
Clarity is the clearness of understanding and expression which should characterize scientific explanations of causality. Thoughts and descriptions should be as lucid and free of ambiguity or indistinctiveness as is possible.
If design analysis were applied to the analysis of living things, how could it constructively add clarity in assessments?
- A researcher would start from the position of asking himself how he would have to design/build every capability into the desired object. If the designer fails to design some capacity into the object that capacity is totally lost. Clarity is enhanced by this activity since it forces the researcher to think about each essential part in a process and not omit from their explanations of causality key components of the entity.
- Designers make plans for distinct entities delineating definite boundaries of “self” from “non-self.”
- If a purpose of the entity is to work together with other entities or inanimate conditions, (in that they are desired to “interact” with each other) that capacity must be designed into each distinct entity. Thus, the designer must program into each entity a logical “if (+/-) x external condition then (+/-) z internal response” which will define for that entity specific parameters initiating its own internal functions.
- If a designer desires an entity to be able to adapt to external conditions, she must build those capacities into the entity enabling it to self-adjust. Thus, it is not truly possible to have an entity “shaped by its environment” since any mechanisms to allow the “shaping” must be designed into the entity ahead of time.
- Analogies which have identifiable equivalency of parts between those of man-made and living things are characteristic of design analysis. This realism brings clarity to descriptions of function by comparing tangible parts, processes, etc.
- But, in terms of the accurate attribution of causality, design analysis is free of anthropomorphisms or metaphors to unreal things, analogies lacking equivalent correlating parts, and mystical language.
- Designers do not rely on any transfer of information from inanimate external conditions to/into the designed entity to “instruct” it in any way. Inanimate things do not possess information in and of themselves and cannot transfer it.
Design Analysis Explains Differential Outcomes
Many researchers prefer the term “differential reproduction” as a synonym to “natural selection.” Why? It is because what is important in terms of long-term adaptation is not “survival” but reproduction. All recognize that unless traits suitable to survival of an organism are passed on to the next generation they contributed nothing to an enduring adaptive outcome. (Darwin would of course disagree since in his conception of natural selection the “struggle for existence” was the crucial event “selecting” the “fittest” traits—assuming that survivors would be able to find mates to reproduce—and would prefer the term “differential survival.”) Differential reproduction may be better explained through design analysis.
Differential successes are anticipated to result from the engineering design process. No one expects that all design solutions will be equally successful in solving a problem—and some are even expected to fail. Causality of the design and, therefore, credit or blame rests solely with the designer. There is a real “success event” related to the design which can actually be observed (in contrast to the mystical “selection event” purported to reside in the environment) which is explained in terms of the innate attributes of the design to solve a problem.
As solutions relate to problems, the fact that engineers may not be able to select in advance which designs will be successful—meaning that there is a contingency—in no manner mystically confers volitional “selective” capacity (and credit) over to the problem to “select” its solution. In addition, problems, which are often environmental conditions, also do not act like a “screen” filtering out the failed designs and cannot be given credit for that role either (no more than a difficult math test can be given credit to screen out ill-prepared from well-prepared students—the test is just an unconscious exposure to students who screen out themselves based on their preparations . . . of which some might want to blameshift to the test).
Amongst living things, differential reproduction is an effect which, in and of itself, explains nothing about causality of that outcome. The cause of the differences may be best explained by the differing genetic/epigenetic information (expressed in differing developmental conditions) resulting in different traits which either succeed or fail to solve the problems of environmental conditions.
Design analysis shows how the Fall does not necessitate any design change to adaptive capacity. Suppose that the true cause of organisms filling niches is solely their innate self-adjusting capability. Suppose further that this capability started so robust that it always possessed the potential to solve perilous, new challenges (like cooperative relationships perverted into predator-prey) in the death-filled, post-Fall world—a function which it was never originally (and still isn’t) purposed to do.
Design Analysis Exposes the Illegitimate Analogy of “Artificial Selection” to “Natural Selection”
Natural selection’s intrinsic spiritual problem was derided by non-theist observers from the outset. In 1864, a perpetual secretary of the French Academy of Sciences, Jean Pierre Flourens, described Darwin’s Origin of Species as “metaphysical jargon thrown amiss in the natural history,” “pretentious and empty language!,” “puerile [silly] and supernatural personifications!,” and stated that Darwin “imagines afterwards that this power of selecting which he gives to Nature is similar to the power of man” (Flourens 1864, p. 65 as quoted in Huxley 1894, pp. 99–100). These critical condemnations of Darwin’s magical phrase “natural selection” also highlight a qualitative difference between semantic reification and an outright personification of that which is, in reality, impersonal.
Eminent Scottish philosopher of biology, Edward Stuart Russell, by 1954 (the year of his death) said, “It is important to note that in all these processes there is no ‘selection’ in the proper meaning of the word. It is unfortunate that Darwin ever introduced the term ‘natural selection’, for it has given rise to much confusion of thought. He did so, of course, because, he arrived at his theory through studying the effects of selection as practiced by man in the breeding of domesticated animals and cultivated plants. Here [within the agricultural context of intelligent, decision-making animal breeders] the use of the word is entirely legitimate. But the action of man in selective breeding is not analogous to the action of ‘natural selection’, but almost its direct opposite, as Woltereck (1931) in particular has pointed out. Man has an aim or an end in view; ‘natural selection’ can have none . . . Nothing of this kind happens, or can happen, through the blind process of differential elimination and differential survival which we miscall ‘natural selection’” (Russell 1962, p. 124).
Familiar claims to the contrary notwithstanding, Darwin didn’t manage to get mental causes out of his account of how evolution works. He just hid them in the unexamined analogy between selection by breeding and natural selection. (Fodor and Piattelli-Palmarini 2010, p. 162, emphasis mine)
Naturalists like to restrict themselves to natural explanations—except when the most natural explanation indicates that causality is not natural.
Design Analysis Reveals Two Key Rescuing Devices of Environmentalists
Certain elements of the environmentalist worldview may be classified as rescuing devices to shield it from the evidence flowing from design analysis that: 1) environments represent inanimate conditions, and 2) problem solving resides in the information-based side of organism-environmental interface. Commenting on characteristics of worldviews, astrophysicist Jason Lisle said, “A clever person will always be able to invent a rescuing device to protect his worldview from what appears to be contrary evidence. His justification for doing this will be the truth of his worldview” (Lisle 2013, p. 73).
1. Environmental agency: Why environmentalists make this appeal
Recall that Meyer documented how philosophers define “agent causation” in the form of conscious, rational intelligent agency. Consciousness, volition, and intelligence have only been observed as attributes of some living things which actually exercise agency. But an environmentalist’s worldview will not allow him to draw that conclusion. His worldview requires environmental causation.
The problem for evolutionary theory is that in all of human experience, when we observe information-based, complex organized systems working together, they are always the product of intelligence, volition, forethought, selectivity, etc. which are also activities in the intelligent design process (Dembski and Wells 2008; Gitt, Compton, and Fernandez 2011). Ascribing volitional agency to inanimate things, as defined by Webster, would make them fetishes or idols and to nature itself a form of animism.
The article, Darwin’s sacred imposter: How natural selection is given credit for design in nature (Guliuzza 2011), documented how natural selection is used in evolutionary theory as the substitute for God’s intelligent design; with the key word being “selection.” It explained why evolutionary literature is inherently filled with volitional words mystically ascribed to environmental conditions such as, “select for/against,” “favor,” “punish,” or “agency.”
In order to protect their worldview from potentially contrary evidence an environmentalist or selectionist may advance this hypothesis: environments really do exercise agency (possibly on behalf of God Himself.) This agency is proposed to be expressed in at least one way; through the claim that environments really do select organisms as evidenced by the observation that some organisms are alive and some dead after an environment acts on them. The observable results show agency, even though volition or intelligence themselves are as yet undetectable by observation.
There is no observational evidence that environments possess consciousness, volition, and intelligence. But then again, their undetectable nature means that agency in environments cannot be disproved at the moment. In like manner a mysticist who trips in the forest may claim that an invisible leg tripped him—despite no evidence for the leg, it cannot be disproved because it is invisible. The environmentalist appeals to his worldview as the justification for his belief in environmental agency.
2. Random genetic mutations: Why they have a universal appeal to environmentalists
Have you ever wondered why evolutionists have historically given so much weight to random genetic mutation? One reason goes back to the basic theme of causality and credit. When one examines adaptation at the organism-environment interface all of the incredibly elaborate systems (which appear intricately designed) to produce the variability, reproduction, and heritability necessary for the expression of adaptation—are innate to the organism. The environment does not have anything close to any identifiable or complete mechanisms for adaptation. If mechanisms existed in the environment they would be described in scientific literature.
Appeals to mutation are a desperate rescuing device by adherents of the environmentalist worldview to find at least something—no matter how counterintuitive due to impotence or destructiveness—that the environment can “due to” organisms ascribe some type of adaptive “causality” to nature itself.
There are at least two problems with this appeal.
First, the advent of a mutation must be set in the context of an organism’s elaborate innate systems, and it is, thus, just as insufficient to explain adaptation as an isolated software glitch explains the self-adjusting functions of an entire rocket.
Second, selectionists overlook the fact that most organisms also have designed mechanisms for preventing and correcting mutation (for example due to ultraviolet light) (González Besteiro et al. 2011, p. 727; Menton 2010, p. 69). Always attuned to the function of systems, Shapiro adds, “We can think of this two level proofreading process as equivalent to a quality-control system in human manufacturing. Like human quality-control systems, it is based on surveillance and correction (cognitive processes) rather than mechanical precision . . . . Another common misperception in many conventional discussions of genomic change is that cells cannot avoid the automatic production of mutations in response to DNA-damaging agent such as UV radiation or mutagenic chemicals. This misperception results from ignorance about the sophisticated apparatus that even the smallest cells possess to repair genome damage and a failure to appreciate the power of cellular genome surveillance and response regimes” (Shapiro 2011, pp. 14–15).
Potential phenotypic changes resulting from mutated DNA could, thus, be properly seen as caused by a failure of the organism’s innate designs in the face of an environmental exposure.
Nevertheless, when upholding natural selection in lieu of design-based explanations, the appeal of evolutionary or theistic selectionists to mutation as a “causative mechanism” is a certainty since they have nothing else.
Conclusion
The examples presented in this paper only illustrate the innate self-adjusting capacity of a few organisms that likely is a basic characteristic of all creatures right from the beginning.
As noted in part 1, this incredible innate capacity is a phenomenon which several top researchers have noted, “For the past century, ecologists and evolutionary biologists have documented the widespread occurrence of phenotypic plasticity, from protists and bacteria to plants and animals” (Relyea 2005, p. 856, emphasis added). Gilbert and Epel agree, “Therefore, a complete list of organisms with phenotypic plasticity would resemble a survey of all eukaryotes on the Tree of Life” (Gilbert and Epel 2009, p. 13). These remarkable observations are from evolutionary scientists about the resilient design of organisms created to “fill” a dynamic and challenging earth, but this is an area where creationists should be leading the way.
References
Abzhanov, A., M. Protas, B. R. Grant, P. R. Grant, and C. J. Tabin. 2004. Bmp4 and morphological variation of beaks in Darwin’s finches. Science 305, no. 5689:1462–1465.
Abzhanov, A., W. P. Kuo, C. Hartmann, B. R. Grant, P. R. Grant, and C. J. Tabin. 2006. The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature 442:563–567.
Agrawal, A. A., J. K. Conner, M. T. J. Johnson, and R. Wallsgrove. 2002. Ecological genetics of an induced plant defense against herbivores: Additive genetic variance and costs of phenotypic plasticity. Evolution 56, no. 11:2206–2213.
Agrawal, A.A., A. P. Hastings, M. T. J. Johnson, J. L. Maron, and J.-P. Salminen. 2012. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338, no. 6103:113–116.
Alon, U. 2007. Network motifs: Theory and experimental approaches. Nature Reviews Genetics 8:450–461.
Andrew, R. L., R. Peakall, I. R. Wallis, J. T. Wood, E. J. Knight, and W. J. Foley. 2005. Marker-based quantitative genetics in the wild?: The heritability and genetic correlation of chemical defenses in eucalyptus. Genetics 171, no. 4:1989–1998.
Anway, M. D., A. S. Cupp, M. Uzumcu, and M. K. Skinner. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, no. 5:1466–1469.
Attig, L., A. Vigé, A. Gabory, M. Karimi, A. Beauger, M.-S. Gross, A. Athias, et al. 2013. Dietary alleviation of maternal obesity and diabetes: Increased resistance to diet-induced obesity transcriptional and epigenetic signatures. PLoS ONE 8, no. 6: e66816.
Beldade, P., P. M. Brakefield, and A. D. Long. 2002. Contribution of Distal-less to quantitative variation in butterfly eyespots. Nature 415, no. 6869:315–318.
Bourdeau, P. E., and F. Johansson. 2012. Predator-induced morphological defences as by-products of prey behaviour: A review and prospectus. Oikos 212, no. 8:1175–1190.
Bowman, R. I. 1961. Morphological differentiation and adaptation in the Galapagos finches. Berkley, California: University of California Press.
Bygren, L. O., G. Kaati, and S. Edvinsson. 2001. Longevity determined by ancestors’ nutrition during their slow growth period. Acta Biotheoretica 49, no. 1:53–59.
Cabej, N. R. 2005. Neural control of development: The epigenetic theory of heredity. Dumont, New Jersey: Albanet.
Cabej, N. R. 2011. Neural control of gene recruitment in metazoans. Developmental Dynamics 240, no. 1:1–8.
Cabej, N. R. 2013. Building the most complex structure on earth: An epigenetic narrative of development and evolution of animals (Elsevier Insights). New York, New York: Elsevier Publishing.
Carroll, S. B. 2005. Endless forms most beautiful: The new science of evo devo. New York, New York: W. W. Norton & Company.
Chong, S., and E. Whitelaw. 2004. Epigenetic germline inheritance. Current Opinions in Genetics and Development 14, no. 6:692–696.
Crain, D. A. and L. J. Guillette. 1998. Reptiles as models of contaminant-induced endocrine disruption. Animal Reproduction Science 53 no. 1–4:77–86.
Cullen, D. A., G. A. Sword, T. Dodgson, and S. J. Simpson. 2010. Behavioural phase change in the Australian plague locust, Chortoicetes terminifera, is triggered by tactile stimulation of the antennae. Journal of Insect Physiology 56, no. 8:937–942.
Dembski, W. A., and J. Wells. 2008. The design of life, pp. 168–170. Dallas, Texas: The Foundation for Thought and Ethics.
Denver, R., N. Mirhadi, and M. Phillips. 1998. Adaptive plasticity in amphibian metamorphosis: Response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 79, no. 6:1859–1872.
Finnegan, E. J., and E. Whitelaw. 2008. Leaving the past behind. PLoS Genetics 4, no. 10: e1000248.
Flourens, J. P. 1864. Examination of the book of Mr. Darwin On the Origin of Species, p. 65. Quoted in T. H. Huxley, Darwiniana, 1894. New York, New York: D. Appleton and Company.
Fodor, J., and M. Piattelli-Palmarini. 2010. What Darwin got wrong. New York, New York: Farrar, Straus and Giroux.
Fornoni, J., P. L. Valverde, and J. Núñez-Farfán. 2003. Quantitative genetics of plant tolerance and resistance against natural enemies of two natural populations of Datura stramonium. Evolutionary Ecology Research 5, no. 7:1049–1065.
Gerstein, M. B., A. Kundaje, M. Hariharan, S. G. Landt, K. K. Yan, C. Cheng, X. J. Mu, et al. 2012. Architecture of the human regulatory network derived from ENCODE data. Nature 489, no. 7414:91–100.
Gilbert, S. F. and D. Epel. 2009. Ecological developmental biology: Integrating epigenetics, medicine, and evolution. Sunderland, Massachusetts: Sinauer Associates.
Gitt, W., B. Compton, and J. Fernandez. 2011. Without excuse. Atlanta, Georgia: Creation Book Publishers.
Godwin, J., J. A. Luckenbach, and R. J. Borski. 2003. Ecology meets endocrinology: Environmental sex determination in fishes. Evolution and Development 5, no. 1:40–49.
González Besteiro, M. A., S. Bartels, A. Albert, and R. Ulm. 2011. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. The Plant Journal 68, no. 4:727–737.
Guliuzza, R. 2011. Darwin’s sacred imposter: How natural selection is given credit for design in nature. Acts & Facts 40, no. 7:12–15. http://www.icr.org/article/darwins-sacred-imposter-how-natural./
Guliuzza, R. 2012. Engineered adaptability. Acts & Facts 41, no. 10:11–14. http://www.icr.org/article/engineered-adaptability/.
Hobbs, J.-P. A., P. L. Munday, and G. P. Jones. 2004. Social induction of maturation and sex determination in a coral reef fish. Proceedings of the Royal Society B London 271, no. 1553:2109–2114.
Jablonka, E., and M. J. Lamb. 2010. Transgenerational Epigenetic Inheritance. In Evolution—the extended synthesis, ed. M. Pigliucci and G. B. Müller, pp. 137–174. Cambridge, Massachusetts: The MIT Press.
Jeanson, N. 2013. Does natural selection exist? A critique of Randy Guliuzza’s claims. Answers Research Journal 6:285–292.
Kaati, G., L. O. Bygren, and S. Edvinsson. 2002. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. European Journal of Human Genetics 10, no. 11:682–688.
Kirschner, M. W., and J. C. Gerhart. 2005. The plausibility of life. New Haven, Connecticut: Yale University Press.
Kraft, P. G., C. E. Franklin, and M. W. Blows. 2006. Predatorinduced phenotypic plasticity in tadpoles: Extension or innovation? Journal of Evolutionary Biology 19, no. 2:450–458.
Lang, M., S. Murat, A. G. Clark, G. Gouppil, C. Blais, L. M. Matzkin, E. Guittard, et al. 2012. Mutations in the neverland gene turned Drosophila pachea into an Obligate specialist species. Science 337, no. 6102:1658–1661.
Lightner, J. K. 2012. Finch beaks point to a Creator who provides. Journal of Creation 26, no. 2:8–10.
Lisle, J. 2013. Young earth presuppositionalism, Christian Apologetics Journal 11, no. 2:1–185.
Mayer, B. J., M. L Blinov and L. M. Loew. 2009. Molecular machines or pleiomorphic ensembles: Signaling complexes revisited. Journal of Biology 8:81.1–81.8.
McCollum, S. A. and J. Van Buskirk. 1996. Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50, no. 2:583–593.
McCollum, S. A., and J. D. Leimberger. 1997. Predator-induced morphological changes in an amphibian: Predation by dragonflies affects tadpole shape and color. Oecologia 109, no. 4:615–621.
Menton, D. A. 2010. Melanin—Umbrellas of our skin. Answers 5 no. 4: 68–70.
Murdock, C., and T. Wibbels. 2006. Dmrt1 expression in response to estrogen treatment in a reptile with temperature-dependent sex determination. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 306, no. 2:134–139.
Newman, R. A. 1992. Adaptive plasticity in amphibian metamorphosis. BioScience 42, no. 9:671–678.
Newman, R. A., and A. E. Dunham. 1994. Size at metamorphosis and water loss in a desert anuran (Scaphiopus couchii). Copeia 1994, no. 2:372–381.
Nijhout, H. F. 1999. Control mechanisms of polyphenic development in insects. BioScience 49, no. 3:181–192.
Nijhout, H. F. 2003. Development and evolution of adaptive polyphenisms. Evolutionary Development 5, no. 1:9–18.
Pembrey, M. E., L. O. Bygren, G. Kaati, S. Edvinsson, K. Northstone, M. Sjöström, and J. Golding. 2006. Sex-specific, male-line transgenerational responses in humans. European Journal of Human Genetics 14, no. 2:159–166.
Pigliucci M. 2003. Epigenetics is back! Hsp90 and phenotypic variation. Cell Cycle 2, no. 1:34–35.
Purdom, G. 2006. Is natural selection the same thing as evolution? In The New Answers Book 1, ed. K. Ham, pp. 271–282. Green Forest, Arkansas: Master Books.
Reik, W., W. Dean, and J. Walter. 2001. Epigenetic reprogramming in mammalian development. Science 293, no. 5532:1089–1093.
Relyea, R. A. 2000. Trait-mediated indirect effects in larval anurans: Reversing competition with the threat of predation. Ecology 81, no. 8:2278–2289.
Relyea, R. A. 2005. The heritability of inducible defenses in tadpoles. Journal of Evolutionary Biology 18, no. 4:856–866.
Richards, E. J. 2006. Inherited epigenetic variation—revisiting soft inheritance. Nature Reviews Genetics 7:395–401.
Richards, E. J. 2011. Natural epigenetic variation in plant species: A view from the field. Current Opinion in Plant Biology 14, no. 2:204–209.
Rogers, S. M., T. Matheson, E. Despland, T. Dodgson, M. Burrows, and S. J. Simpson. 2003. Mechanosensory-induced behavioural gregarization in the desert locust Schistocerca gregaria. Journal of Experimental Biology 206, no. 22:3991–4002.
Rohner, N., D. F. Jarosz, J. E. Kowalko, M. Yoshizawa, W. R. Jeffery, R. L. Borowsky, S. Lindquist, and C. J. Tabin. 2013. Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342, no. 6164:1372–1375.
Ruden, D. M., M. D. Garfinkel, V. E. Sollars, and X. Lu. 2003. Waddington’s widget: Hsp90 and the inheritance of acquired characters. Seminars in Cell & Developmental Biology 14, no. 5:301–310.
Ruden, D. M., and X. Lu. 2008. Hsp90 affecting chromatin remodeling might explain transgenerational epigenetic inheritance in Drosophila. Current Genomics 9, no. 7:500–508.
Russell, E. S. 1962. The diversity of animals: An evolutionary study. Leiden, Germany: E. J. Brill.
Rutherford, S. L., and S. Lindquist. 1998. Hsp90 as a capacitor for morphological evolution. Nature 396, no. 6709:336–342.
Sangster, T. A., N. Salathia, S. Undurraga, R. Milo, K. Schellenberg, S. Lindquist, and C. Queitsch. 2008. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proceedings of the National Academy of Sciences, USA 105, no. 8:2963–2968.
Shapiro, J. A. 2011. Evolution: A view from the 21st century. Upper Saddle River, New Jersey: FT Press Science.
Shoemaker, C., M. Ramsey, J. Queen, and D. Crews. 2007. Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Developmental Dynamics 236, no. 4:1055–1063.
Singh, J., M. Freeling, and D. Lisch. 2008. A position effect on the heritability of epigenetic silencing. PLoS Genetics 4, no. 10: e1000216.
Thomas, B. 2011. Fish designed to tolerate poison. Institute for Creation Research. Accessed November 25, 2013. http:// www.icr.org/article/fish-designed-tolerate-poison/.
Thomas, B. 2009. The confusing origin of locust swarms. Institute for Creation Research. Accessed November 25, 2013. http://www.icr.org/article/confusing-origin-locust-swarms/.
Thomas, B. 2014. Birds’ built-in defenses fend off radiation. Institute for Creation Research. Accessed May 12, 2014. http://www.icr.org/article/8063/.
Van Buskirk, J., and S. McCollum. 2000. Influence of tail shape on tadpole swimming performance. The Journal of Experimental Biology 203: 2149–2158.
Van Buskirk, J. 2001. Specific induced responses to different predator species in anuran larvae. Journal of Evolutionary Biology 14, no. 3: 482–489
Warner, R. R., and S. E. Swearer. 1991. Social control of sex change in the Bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae). The Biological Bulletin 181, no. 2:199–204.
Waterland, R. A. and R. L. Jirtle. 2003. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Molecular and Cellular Biology 23, no. 15:5293–5300.
Waterland, R. A., M. Travisano, and K. G. Tahiliani. 2007. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB Journal 21, no. 12:3380–3385.
Weaver, I. C., A. C. D’Alessio, S. E. Brown, I. C. Hellstrom, S. Dymov, S. Sharma, M. Szyf, and M. J. Meaney. 2007. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: Altering epigenetic marks by immediate-early genes. Journal of Neuroscience 27, no. 7:1756–1768.
Woodward, D. E. and J. D. Murray. 1993. On the effect of temperature-dependent sex determination on sex ratio and survivorship in crocodilians. Proceedings of Royal Society of London B, 252, no. 1334:149–155.
For reference:
- Jeanson’s 2013 paper: Does Natural Selection Exist?
- Guliuzza’s response 1: A Response to “Does Natural Selection Exist?”: Creatures’ Adaptation Explained by the Design-based, Organism-Driven Approach: Part 1
- Guliuzza’s response 2: A Response to “Does Natural Selection Exist?”: Creatures’ Adaptation Explained by the Design-based, Organism-driven Approach: Part 2
- Guliuzza’s response 3: A Response to “Does Natural Selection Exist?”: Creatures’ Adaptation Explained by the Design-based, Organism-driven Approach: Part 3
- Jeanson’s reply: Reply to “A Response to ‘Does Natural Selection Exist?’”