The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
This review of attempts to postulate evolutionary theories for the origin and development of cartilage and the cells that produce cartilage. The review found a complete lack of evidence for both the origin and development of cartilage. Even plausible “just-so” stories have not been attempted by evolutionists. For mammals, especially primates, life would not be possible without cartilage. This is yet another example of a body structure whose evolutionary origin completely baffles Darwinists. They recognize that mammalian life could not survive without cartilage and yet cannot explain how cartilage evolved to be part of the mammal-body design. Cartilage is, therefore, part of the irreducibly complex design of mammals that meets Darwin’s bold 1859 challenge that “If it could be demonstrated that any complex organ existed, which could not possibly have been formed by numerous, successive, slight modifications, my theory would absolutely break down” (Darwin 1859, 189). Although Darwin wrote that he did not know of any such contrary example, cartilage is one of many excellent Darwin-defying candidates.
Introduction
Cartilage is a seemingly simple substance that has scores of important functions in animal bodies. It is used everywhere in the body, even to hold tubes open, such as the cricoid cartilage and the C-shaped tracheal rings below it that traverse the entire length of the trachea and primary bronchi. The cartilage keeps the bones from wearing out by acting as a bushing between them. In addition, synovial fluid greases the joint surfaces allowing for smooth movement. This plastic-like elastic tissue also has a structural function as part of the rib cage, the ear, and the nose. The vertebrate intervertebral discs are made of fibrocartilage that contains an outer collagen ring, the annulus fibrosus, and a central gel, the nucleus polposus (fig. 1).
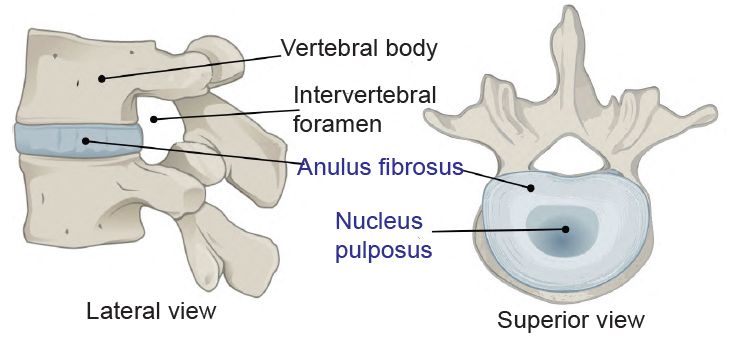
Fig. 1. The intervertebral disk. https://upload.wikimedia.org/wikipedia/commons/3/3c/716_Intervertebral_Disk.svg. https://commons.wikimedia.org/wiki/File:716_Intervertebral_Disk.svg.
In embryos of vertebrates and certain other animals, the first skeletal structural system for most long bones (arms and legs) is cartilage, which is replaced with true bone as development proceeds. The skull and facial bones form from bone directly, not cartilage. Cartilage is avascular (does not contain blood vessels) and aneural (does not contain nerves), thus does not feel pain but also heals very slowly. In short, it is a critical construction material which holds the body together. Development of the three types of cartilage (hyaline, elastic, and fibrocartilage) are controlled by the genetic coding process that reveals itself during embryonic, fetal, and even youth differentiation.
To be labeled cartilage, a connective tissue must meet certain histological criteria that include its composition and the organization of its extracellular matrix (Cole and Hall 2004, 261). The major composition of cartilage includes glycosaminoglycans, proteoglycans, collagen fibers, and sometimes elastin (Cole 2011). The three basic types, hyaline, elastic, and fibrocartilage, complicate the evolution-of-cartilage problem. Hyaline cartilage, the most common type, is used at the ends of bones, lining the joints of the body—as well as in the septum of the nose and part of the respiratory conductive tubing in the lungs. Elastic cartilage, a highly flexible formulation of cartilage, is employed mainly in the ear and nose (fig. 2). Fibrocartilage and hyaline cartilage are used in the knee menisci and the spinal discs. It is far less flexible than elastic cartilage.
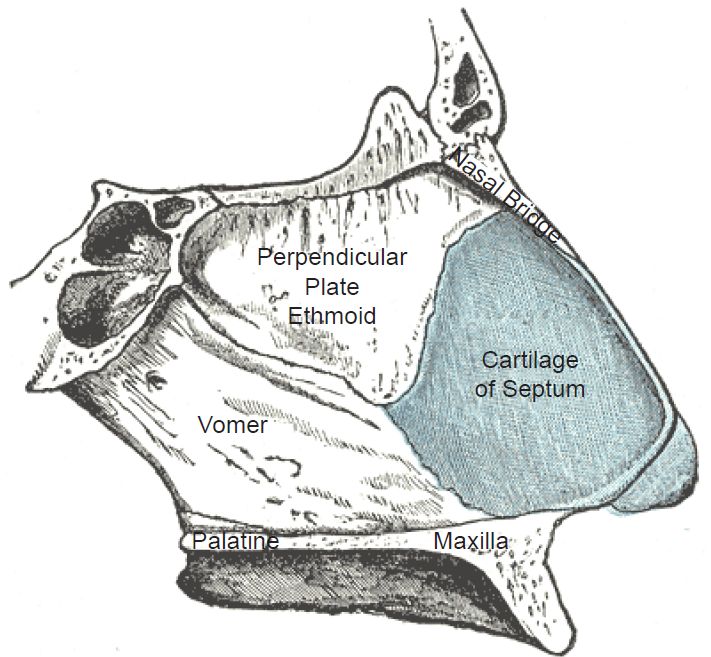
Fig. 2. The cartilage in the human nose. https://en.wikipedia.org/wiki/Nasal_septum#/media/File:Gray854.jpg. Public Domain.
Cartilage is a non-cellular matrix manufactured by cells within the matrix. Specifically, chondroblasts form it and chondrocytes maintain it. Chondroblasts are to cartilage as osteoblasts are to bone. The cartilage ground substance is chondroitin sulfate, and the fibrous sheath surrounding it is perichondrium. The cartilaginous matrix consists mainly of collagen and proteoglycans.
The Evolution of the Chondroblasts and Chondrocytes
To document the evolution of cartilage, the evolution of the chondroblasts must also be documented. After noting that “the evolutionary relationship among skeletal tissues is unclear,” evolutionists speculate that “immature cartilage evolved before mature cartilage or bone” (Gómez-Picos and Eames 2015, 2). Immature cartilage is formed by chondroblasts which deposit a network of loose collagen fibers and a rich substance of proteoglycans. The deposit is matured by decreasing its proteoglycan sulfation and also adding minerals (Gómez-Picos and Eames 2015).
No published evidence exists for the evolution of the chondroblast cell type: “the question of the evolutionary origin of these cell types remains” (Cole 2011, 127). One theory holds that because chondrocytes in different life-forms are significantly different, chondrocytes in different lineages are the result of independent, convergent evolution. Although no viable “just-so” stories have ever been proposed for the evolution of the first functional chondroblast, evolutionists propose that they must have evolved independently several times to explain the differences!
New research for evidence for evolution of the chondrocyte cell type turns out to be far more elusive than previously imagined. The function and design of this coating that covers other tissues, including on bones at the joint, would seem to be straightforward, but as one study admitted,
Exactly how cartilage manages this near-frictionless, shock absorbing function is not fully understood. It is generally accepted that it depends on interactions between fluid in the joint and the molecules that make up the tissue, known as the extracellular matrix (ECM). Studying these subtle dynamics at the microscopic level has long been a goal of scientists. (Malooley 2022)
One of hyaline cartilage’s major roles is to coat the ends of our bone joints, allowing them to smoothly glide past one another. The surface it creates is about five times more slippery than ice on ice. It also cushions bones, helping to protect them from the force of impact, which can be enormous. In a healthy adult male, the femur can support 30 times the body weight. This equals roughly 6,000 lb of compressive force for the average adult male. Glycoaminoglycans in cartilage attract water, and the more water in the matrix, the greater the cushioning.
A good illustration of design is the hip joint which is made of two different cartilage types. The acetabulum has a C-shaped articular surface, the lunate surface, which is lined by hyaline cartilage. As shown in fig. 3, the rim of the acetabulum is lined with a fibrocartilagenous acetabular labrum, part of which bridges across the acetabular notch as the transverse acetabular ligament.
The pursuit to better understand this misnamed “simple substance” called cartilage has required some of the most complex sophisticated research tools ever developed by mankind. This includes x-ray photon spectroscopy using ultra-bright x-ray beams (Partain et al. 2021). The molecular level evaluated by this test was less than one micron, which is fully 70 times smaller than the width of a single human hair (Malooley 2022). The research goal was to better understand and treat cartilage problems and diseases such as osteoarthrosis.
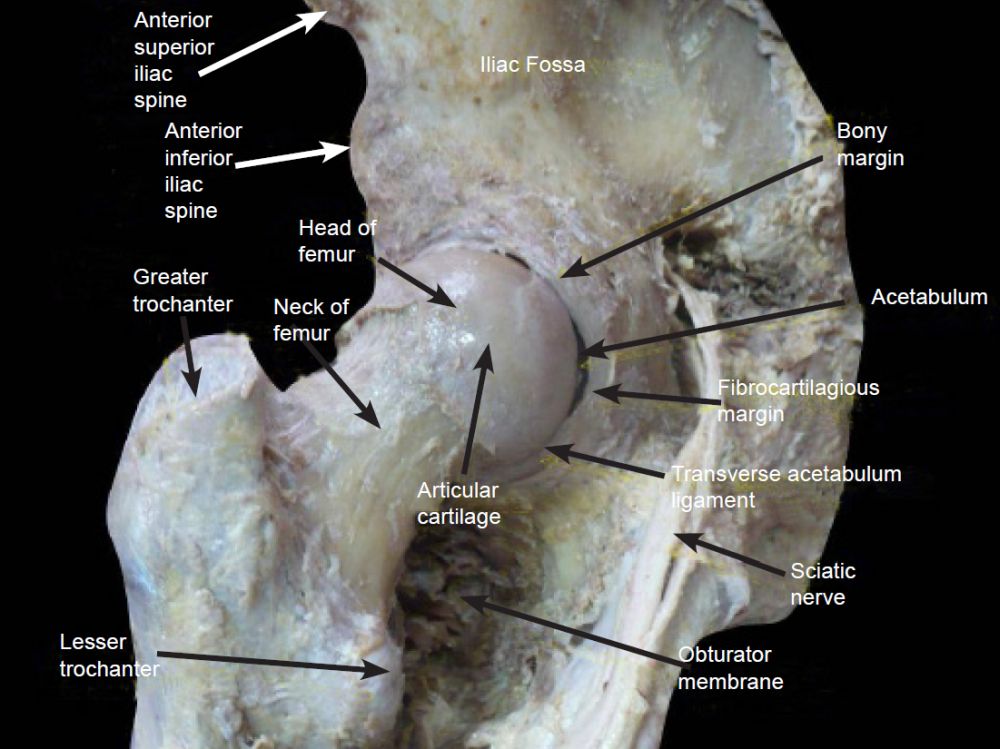
Fig. 3. Acetabulum details. https://www.wikiwand.com/en/Acetabulum.
The study determined that cartilage “behaves as a biphasic material, where material mechanics derives from a fluid phase, [a] solid phase, and interaction between the two” phases (Partain et al. 2021, 1354–1355). Cartilage was found to consist of a complex integration of several materials that function according to the dynamics of the extracellular matrix related to temperature, pressure, and other factors. One example was that, when hydrated,
cartilage matrix dynamics are depth dependent, with matrix dynamics fastest at the cartilage surface and progressively slowing with increased depth . . . . Healthy cartilage has a hierarchical structure that is often described as three different zones: surface zone, middle zone, and deep zone. Each zone has a different structural composition that affects matrix mobility. (Partain et al. 2021, 1354)
In short, cartilage is designed to function very effectively in its many roles and locations in the body. The study concluded that cartilage design illustrates the fact that
tissues have complex structures that provide complex physical functions. These functions derive from interactions occurring across length scales. However, many questions remain in how tissue interactions serve to provide mechanical function. (Partain et al. 2021, 1356)
Most of our skeleton develops from endochondral ossification. Exceptions include the cranial bones and clavicles (collar bones) which grow from intramembranous ossification which allows for natural childbirth.
Cartilage Evolution
Cartilage evolution has long baffled evolutionists: “Our skeletons evolved from cartilaginous tissue, but it remains a mystery how cartilage itself first arose in evolution” (Brunet and Arendt 2016). The evolutionary origin of cartilage itself is widely debated: “Theories of the evolutionary origin(s) of cartilage abound. Most start from the premise that cartilage is exclusively a vertebrate tissue” (Hall 2015, 74). No direct evidence exists for the evolution of this complex substance from simpler substances. It even exists in so-called primitive animals, including a few isolated groups of protostomes (for example, worms, arthropods, mollusks): chelicerates (for example, arachnids, sea spiders, horseshoe crabs) and cephalopods (for example, squid, octopus) (Cole and Hall 2004).
The cartilage composition in a wide variety of animals, from cuttlefish, horseshoe crabs, fish, mammals to humans, is structurally very similar. Animals either utilize cartilage or use some other very different support system such as chitin. Chitin is a tough, protective, semitransparent nitrogen-containing polysaccharide used in many insects. Nothing in between chitin and cartilage, nor any evidence of cartilage evolution from anything else, has ever been documented.
For evolutionists, the scattered distribution of cartilage types and uses is evidence that “cartilage evolved independently in each of these groups and was not present in their last common ancestor” (Brunet and Arendt 2016, R686). The problem with this explanation is that no evidence exists that it evolved even once, so to postulate it evolved numerous times does not help the Darwinists’ case.
Another problem for evolution is that no evidence of a common ancestor exists which first evolved cartilage, and no case exists where all of its supposed descendants contain cartilage exclusive of other animal types of connective tissue. For example, if cartilage first evolved in mammals, one would theorize that it would be used in all mammals and no other life-form. In fact, it is employed not only in all mammals but also in a scattered set of unrelated life-forms, including cartilaginous fish, some invertebrates including horseshoe crabs, some mollusks (marine snails) some cephalopods, and even certain annelids like feather duster worms (sabellid polychaetes). The problem is, it is not used in the assumed common ancestor of cephalopods, crabs, mollusks, or annelids, but appears somewhat randomly in isolated members of these groups. This fact supports the view that it did not evolve but was rather engineered to function in each animal which employs it. It was used in certain animals, but not in other animals of the same family. It is not used in any plant, seed, fungi, or bacteria.
One evolutionary scenario is: “Vertebrate cartilage and bone may have arisen from the same ancestral chondroid connective tissue that gave rise to the invertebrate cartilages” (Cole and Hall 2004, 272). Although animal bones are the heart of fossil research, no evidence exists of bone evolution either. The fact is, “Vertebrates are the only animals that produce bone, but the molecular genetic basis for this evolutionary novelty remains obscure” (Gómez-Picos and Eames 2015). Consequently, for evolutionary biologists
the hard parts of animals are similarly double-faced: their endurance makes them the prime candidates for fossilization and provides paleontologists with a wealth of information on the skeleton of extinct animals. From the paleontologist’s view, animal evolution is thus mainly the evolution of hard parts (plus what can be deduced from them). But for the same reason, the origin of the first animal skeletons, and the ancestral structures that gave rise to them in soft-bodied animals, remains mysterious. (Brunet and Arendt 2016, R685)
Cartilage, as a soft tissue, is not normally preserved in the fossil record, but enough examples exist to conclude that the first examples of cartilage are identical to modern cartilage. The oldest, most complete shark fossil, the cartilaginous Cladoselache, is dated by evolutionists at 360 million years old (Gillis et al. 2011). Dating was based on the lava flows between which the encasing sedimentary rocks were sandwiched. Although the evolution of cartilage is problematic, similar problems also exist with bones. For over
a century, morphologists have been debating, with precious little evidence, the hard questions of skeleton origins: When did animal skeletons first evolve? Did they appear once or several times independently? Which ancestral soft tissues first became rigid, and by what molecular mechanisms? (Brunet and Arendt 2016, R686)
The problem for evolution is cartilage is believed to be the “evolutionary precursor to vertebrate bones.” And the fact that bones show no evidence of evolution does not lend support to the theory that bones evolved from cartilage.
Summary
Not only are the three different types of cartilage with their various distinct functions, including cartilage’s near-friction-less shock-absorbing function, not fully understood, but no evidence of its evolution has been uncovered, either in the fossil record or from genetic studies. Rather, cartilage shows intelligent design which is coded for in our DNA.
Research on cartilage follows the human anatomy trend in that it is a far more complex structure than previously believed. The evidence of the evolution of this complex structure has so far eluded evolutionists, as has the evolution of bone from cartilage. The explanation attempted by evolutionists is that it evolved much farther back in the fossil record than we have evidence for (Hall 2015). The problem with this claim is that, among living animals, no pattern of cartilage used only in certain animals, such as higher vertebrates, has ever been found. It appears scattered in animal life from some very primitive organisms to many of the most advanced life forms. This is in dynamic contrast to what is expected, namely its non-existence in the lowest life-forms to its being increasingly more common as we go up the putative evolutionary ladder.
Nobel Laureate Christian de Duve, in his book, attempted to explain singularities. A singularity is a unique event that likely only happened once in the evolution of life, such as the origin of cartilage. Professor de Duve lists several possibilities that explain singularities, including a frozen accident, fantastic luck, and intelligent design. Intelligent design “. . . postulates the occurrence of evolutionary steps that could not possibly have taken place without the intervention of some kind of supernatural guiding entity . . . [and which] can come into account only after all natural explanations have been ruled out . . . .” (de Duve 2005, 4–5). The evolutionary origin of cartilage is one of many well researched examples supporting this view.
References
Brunet, Thibaut, and Detlev Arendt. 2016. “Animal Evolution: The Hard Problem of Cartilage Origins.” Current Biology 26, no. 14 (25 July): R667–R688. https://www.cell.com/current-biology/pdf/S0960-9822(16)30562-0.pdf.
Cole, Alison G. 2011. “A Review of Diversity in the Evolution and Development of Cartilage: The Search for the Origin of the Chondrocyte.” European Cells and Materials 21, (February): 122–129.
Cole, Alison G., and Brian K. Hall. 2004. “The Nature and Significance of Invertebrate Cartilages Revisited: Distribution and Histology of Cartilage and Cartilage- Like Tissues Within the Metazoa.” Zoology 107, no. 4 (December): 261–273.
Darwin, C. 1859. On the Origin of Species by Means of Natural Selection. London, United Kingdom: John Murray.
de Duve, Christian. 2005. Singularities: Landmarks on the Pathways of Life. New York, New York: Cambridge University Press.
Gillis, J. Andrew, Kate A. Rawlinson, Justin Bell, and Neil H. Shubin. 2011. “Holocephalan Embryos Provide Evidence for Gill Appendage Reduction and Opercular Evolution in Cartilaginous Fishes.” Proceedings of the National Academy of Sciences 108, no. 4 (January 10): 1507–1512.
Gómez-Picos, Patsy, and B. Frank Eames. 2015. “On the Evolutionary Relationship Between Chondrocytes and Osteoblasts.” Frontiers in Genetics 6 (September 23): 297. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4585068/pdf/fgene-06-00297.pdf.
Hall, Brian K. 2015. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. New York, New York: Elsevier Academic Press.
Malooley, Jake. 2022. “Sliding into Place: Study Shows How Cartilage Interacts with the Joints in our Bodies.” Argonne National Laboratory, March 14. https://www.anl.gov/article/sliding-into-place-study-shows-how-cartilage-interacts-with-the-joints-in-our-bodies.
Partain, Brittany D., Qingteng Zhang, Mythreyi Unni, Jessica Aldrich, Carlos M. Rinaldi-Ramos, Suresh Narayanan, and Kyle Allen. 2021. “Spatially-Resolved Nanometer-Scale Measurement of Cartilage Extracellular Matrix Mobility.” Osteoarthritis and Cartilage 29, no. 9 (September): 1351–1361.