The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
Charles Darwin originally proposed sexual selection to account for the maintenance of biological traits that provide no apparent survival benefit to a population, traits like the peacock’s extravagant plumage. In more recent times, the Handicap Hypothesis (the idea that males signal their genetic quality to females by their ability to support showy displays) has been presented as a rationale to explain the origin of such counter-intuitive strategies within the larger Darwinian model of fitness. But prominent critics within the evolutionary community continue to be skeptical of this explanation and theoretical challenges are serious. I review two prominent studies of female preference for ornamentation and propose follow-up research that I believe might experimentally test the Handicap Hypothesis. Within a creationist model, the persistence of such “exaggerated” traits might also seem to be problematic. I hypothesize that there is an underlying genetic mechanism designed to maintain these traits that are “beautiful” but appear to handicap the species. The variability of female trait preferences hints at a complex genetic linkage, balancing the maintenance of beauty against the maximization of survival. This is a hallmark of design. This hypothesis of genetic linkage could be tested in the near future as genetic mapping tools become more precise and powerful.
Introduction
Gavest thou the goodly wings unto the peacocks? or wings and feathers unto the ostrich? (Job 39:13, KJV)
The animal world around us is brimming with color, sound, and ornamentation far beyond that which would reasonably be required for successful biological functionality. A simple dive into a reef reveals a vividly beautiful tapestry exhibited by assorted reef fish, a sensory experience that exceeds reasonable expectations. Who hasn’t marveled at the brilliance exhibited by particular animal species, the crimson cardinal, the betta’s colorful, oversized fins, the gaudy decorations of bowerbird’s nests, or the iridescent blue morpho butterfly? Indeed, maximizing the showy displays of a pet species is oftentimes the delight of breeders. But how did such traits develop in the wild in the first place? Why should there be such “gratuitous beauty”? The plethora of extravagant biological characteristics prominently call out for an explanation. Is this God’s way of making our world interesting? Is this the random outcome of genetic Russian Roulette? Charles Darwin, a pigeon fancier, knew about such showy traits and proposed sexual selection to explain them (Darwin 1858). In more recent times, the Handicap Hypothesis has been presented as the Darwinian rationale behind the origin of sexual selection of traits with no apparent survival benefit (Zhavi 1975). In this paper, I will discuss the challenges inherent in the origin and maintenance of “beautiful” ornamentation within both the Darwinian and the design models.
Sexual Selection
The classic example of an adaptation that has no apparent survival benefit is a peacock’s tail plumage (Fig. 1). Charles Darwin famously wrote to a friend, “The sight of a feather in a peacock’s tail, whenever I gaze at it, makes me sick!” (Darwin 1860, quoted in Curry 2008, 82). Believing that natural selection was not sufficient to explain certain types of non-survival adaptations, Darwin devised the concept of sexual selection (Darwin 1858). He argued that “fitness” in this sense is not measured in terms of survival to produce offspring but in the ability to garner mating opportunities to produce offspring. The less fit might continue to survive, but their genes do not get passed on.

Fig. 1. Male peacock. Azim Khan Ronnie, “Peacock Male,” https://commons.wikimedia.org/wiki/File:Peacock_Male.jpg. CC BY-SA 4.0.
Sexual selection has become a favorite area of study in behavioral ecology. A leading, graduate-level ecology textbook introduces the concept of mating behaviors that reflect the costs and benefits of parental investment and mate defense (Cain, Bowman, and Hacker 2014). Then the text pivots to present specific notions of sexual selection. Some of these cases of sexual selection are pretty straightforward. A strong muscular frame (often demonstrated by a fight with other potential mates) generally affords the herd with the best genes for use in mating. This broad principle of finding the strongest individual to utilize in breeding (Fig. 2) is ubiquitous. Male damselflies fight violently to control territories so they can earn a mate. The herd of female elk goes to the reigning male who successfully fights off competitors. The end result is a bigger, stronger breeding male, which doesn’t differ much from the strategy humans employ in selectively breeding their livestock.

Fig. 2. Cades cove deer fighting. Brian Stansberry, “Cade Cove Deer Fighting tn1.jpg,” https://commons.wikimedia.org/wiki/File:Cades-cove-deer-fighting-tn1.jpg. CC BY-SA 3.0.
But what about those absurd displays involving vivid colors, extravagant tail feathers or “useless” ornaments? Unlike large, muscular bodies or powerful fighting weapons these traits offer no obvious survival advantage to the individual—and some pretty apparent disadvantages. Predators are easily drawn to colorful displays; excess plumage actually hinders flight; and there are significant costs to producing and maintaining such extraneous ornamentation. If sexual selection of these “fitness reducing” traits is a widespread biological phenomenon, it would seem to fit better within a design framework than an evolutionary, survival-of-the-fittest framework. The Nobel prize-winning American biologist Thomas Hunt Morgan wrestled through this issue and the difficulties prompted him to abandon the whole idea of sexual selection: “the theory meets with fatal objections at every turn” (Andersson 1994, 18). So how do evolutionary theorists believe that such a non-intuitive method of choosing a desirable mate came to exist in the first place?
The Handicap Hypothesis
Cain, Bowman, and Hacker (2014) answer this question with a concept known as the Handicap Hypothesis, which is the idea that ornamentation is a product of superior genetics and somehow the female recognizes that. The Hypothesis was originally formulated in 1975 by the Israeli biologist Amotz Zahavi:
It is suggested that characters which develop through mate preference confer handicaps on the selected individuals in their survival. These handicaps are of use to the selecting sex since they test the quality of the mate. The size of characters selected in this way serve as marks of quality. The understanding that a handicap, which tests for quality, can evolve as a consequence of its advantage to the individual, may provide an explanation for many puzzling evolutionary problems (Zahavi 1975, 205).
The British geneticist John Maynard Smith was an early skeptic of the Handicap Hypothesis (Smith 1976), sighting the disadvantages incurred by sons. He proposed instead a “revealing handicap” (Smith 1985) in which honest communication of genetic health is not maintained by a cost but by an inescapable correlation between the nature of the signal and the signaler’s quality. Since it really isn’t a “handicap,” Smith’s idea was renamed “The Index.” The principle is that the signal itself is displayed in greater clarity in healthier males. Perhaps the weaker peacock still has a prominent tail, but may struggle to hold it up. As the tail drags, it naturally becomes dull and bug infested. Although Smith discussed the peacock, the costly signal of a goat’s horns, an elaborate bird song or a captured prey offered as a gift to the female can all fit the paradigm. I will further discuss Smith’s Index below.
Other evolutionists were even more critical of Zahavi’s work. For example, Kirkpatrick (1986) said that the Hypothesis would likely cause a serious reduction in average survival rate in the population and so the female mating preferences must involve some other selective force at work. But an important paper by Grafen (1990) revived the popularity of the Handicap Hypothesis by presenting a mathematical model that demonstrated how a costly display could establish a stable communications paradigm. These theoretical models became the basis for “game theory,” whereby each organism is forced to honestly communicate its fitness. The models were said to clarify Zahavi’s Handicap principle and show him to have been substantially correct about its importance and scope. Grafen even included a scenario in which the cost of the extravagant signal is completely paid for by the females via their progeny receiving better-quality genes.
Today the Hypothesis fits into a realm of evolutionary biology known as animal signaling. It is hypothesized that females evolved mating preferences for males who display ornaments that are costly to develop and maintain. This costliness necessitates them having high genetic quality, which is called an “honest” signal (as opposed to bluffing or sending a dishonest signal just to obtain mating opportunities). The female then takes note of the honest signal (sexual selection). By choosing the more desirable male in this way, quality genes can be passed on to the next generation. “Lower quality” males are thought to be weeded out of the population by means of sexual selection, in addition to the normal mechanisms of natural selection. Fig. 3 displays a graphical representation of a Zahavian Handicap (after Johnstone 1995) where CL is cost to a signaler with low-quality genes and CH is cost to a signaler with high-quality genes. Optimal signaling levels are SL for low-quality and SH for high-quality males. Modern Darwinian game theorists have sought to construct mathematical models involving biological signaling whereby honesty can prevail, leading to a stable communications paradigm. Although it has been called one of the most influential ideas in evolutionary biology (Huttegger, Bruner, and Zollman 2015), still today not everyone in the evolutionary community is convinced by the Handicap Hypothesis.
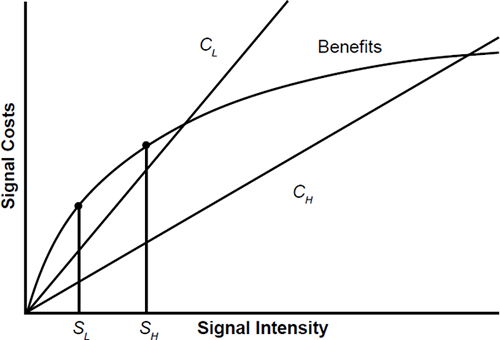
Fig. 3. Modeling the Handicap Hypothesis (after Johnstone 1995).
Ongoing Skepticism of the Handicap Hypothesis
It seems that signaling is ubiquitous in the biological world, from hatchling birds crying out for food, to the gaudy male peacock spider’s courtship display to the stotting gazelle seeking to turn away a predator by his repeated leaping. Zahavi and his proponents applied the Handicap Hypothesis to explain all of these scenarios (Zahavi and Zahavi 1997). Yet there continues to be prominent critics from within the Darwinian community who are opposed to the Hypothesis. Grose (2011) pointed out that acceptance of the Handicap principle has been driven principally by theoretical modeling rather than by empirical results. This is especially troubling because certain empirical studies have failed to find the significant signal cost postulated by the handicap mechanism (Kane and Zollman 2015). A number of “poster-child species” have, upon rigorous testing, failed to confirm the predictions of sexual selection theory (Roughgarden and Akçay 2010).
Not only have observations failed to confirm the Handicap Hypothesis, it faces ongoing theoretical challenges: “the handicap principle faces serious problems from both empirical . . . and theoretical perspectives” (Huttegger, Bruner, and Zollman 2015). “Much of the theoretical work of the past several decades has focused on demonstrating the theoretical coherence of the Handicap Principle in a number of different types of interactions. As a result, the theory has come to occupy a central place in biological theory despite both empirical and theoretical concerns” (Zollman 2013). One of the theoretical concerns is whether honest signaling really even requires a large cost. Lachmann, Számadó, and Bergstrom (2001) constructed models of cost-free signaling equilibria using Grafen’s own paradigm.
Other challenges involve population genetics. Consider beneficial mutations. What if a peacock enjoyed a rare beneficial mutation that gave him an increased chance of surviving predation? How would he “know” about it to send an honest signal that he had “good genes” when his immediate health situation wouldn’t be improved? Might not sexual selection be running directly up against natural selection? Another genetic hurdle still not resolved in the literature involves the loss of beneficial diversity that would result from prominent sexual selection (called the “lek paradox”). Finding the Handicap Hypothesis unconvincing, some researchers have proposed replacing the whole model of sexual selection. “Since its proposal, problems with this narrative have continued to accumulate, and it is our view that sexual selection theory needs to be replaced. We suggest an approach that relies on the exchange of direct ecological benefits among cooperating animals without reference to genetic benefits” (Roughgarden, Oishi, and Akçay 2006). But I believe a more fundamental theoretical challenge to the Hypothesis must be addressed.
Challenging the Origin of a Handicap
I would like to go back to consider the earliest stages in the development of an extravagant display. Imagine a scenario. Sara was an ancient peafowl Pavo cristatus (Fig. 4) who received a happenchance genetic mutation giving her the predisposition to favor a certain odd trait in males, a showy tail. She looked around her large peafowl flock to make sure she selected the peacock with the largest tail for her mate (Fig. 2). Because he happened to possess “good” genes, her brood survived. Her daughters thereafter had a high likelihood of receiving that allele for “choosiness.” In this way, female mate selectivity began multiplying in the population. Her sons, along with any other offspring of that male, likely displayed the big tail trait. “Runaway sexual selection” was suddenly underway such that subsequent generations would experience pressure for ever larger and more gaudy tails (Fisher 1930).

Fig. 4. Indian peafowl (peahen). https://www.canstockphoto.com/female-indian-peafowl-isolated-over-20208840.html.
But we should pause at this point and ask how this system of females preferring a showy tail and males growing ever bigger and more colorful tail displays necessarily correlate with actual genetic health. Zahavi would have us believe that an individual with a prominent deleterious trait communicates that he must have “good” genes to have survived despite this hindrance. But how are “good” genes defined or actually measured? We might say that, by definition, a deleterious trait like a ridiculous tail on an individual means they have “bad” genes. Oftentimes differential survival is about slight advantages and isn’t so dramatic as to suddenly eliminate a deleterious allele. Therefore, odd traits that carry a slight disadvantage persist in the population, with or without any compensating “good” genes. If, as a rule, evident deleterious mutations in males attracted more mates, this would be a one-way ticket to extinction for that population! Think again about our hypothetical peafowl population. Wouldn’t other peahens without Sara’s “mate choosiness” mutation do just as well or even better in the struggle for existence? A different hen who by happenchance received a genetic mutation preferring mates of large size, cryptic coloration, or greater fighting ability should have led her progeny to success in the struggle for existence against Sara’s brood.
It is not possible to go back in time to test if Sara’s mutated descendants would actually outcompete the postulated original wild type, but let’s consider the next step in the development of this trait. Sara’s female preference alleles and her son’s gaudy tail alleles must spread through the local population at the expense of competing traits (like larger body size or cryptic coloration). Ever longer and more brilliant tail feathers must continue to be selected over many generations before the highly nuanced honest signaling system became established and beneficial. But would not Sara’s local population have been at a serious disadvantage as compared to the neighboring peafowl populations that did not pursue this wasteful path? If so, the trait should have gone extinct as migration and encroachment tended to be a one-way street, with genes flowing from the growing neighboring populations of drab peafowl Pavo cristatus and with potentially scarce resources being denied to Sara’s less successful clan. This particular challenge to the Handicap Hypothesis I believe could be tested.
Testing the Handicap Hypothesis
Female preference for ornamentation has been experimentally verified. Andersson (1982) modified the tail feathers of various African long-tailed widowbirds (Euplectes progne) (Fig. 5). Tailfeathers of captured males were significantly cut. Those chopped feathers were then glued to the corresponding feathers of other males to create a “supertail.” Still other males served as controls with their tail left unchanged or with it cut and glued back on. The birds were released and mating success was observed. A pattern emerged where males with the super-elongated tails were the most successful. A deficiency in this research is the lack of long-term data collection involving the survivability of birds with modified tails.

Fig. 5. Male Euplectes progne. https://commons.wikimedia.org/wiki/File:Euplectes_progne_male_South_Africa_cropped.jpg. Public Domain.
Other studies demonstrated that these female choice preferences can morph over time, depending on availability of their preferred mates. Research involving sexual selection in the stalk-eyed fly Cyrtodiopsis dalmanni (Fig. 6) was published by Wilkinson and Reillo (1994). Three lab populations of flies were isolated, a control group, a group of 10 males sporting very long eyestalks, and a group with really short eyestalks. After 13 generations it was shown that females in the population with the long eyestalks preferred males with this trait (as did the control group), but the females in the short eyestalk group actually had changed to prefer short eyestalks. Could we not build on this study and seek to quantify “good genes” somewhat by testing in a competitive natural setting? I would like to see another experiment created to follow up on Wilkinson and Reillo’s work, testing if a population with a short stalk preference enjoyed improved survival vs a population with a long stalks preference. The experiment would require maintaining both populations for some time and gradually introducing them into a homogenous, natural-like environment. This would ascertain how they fared in relation to each other when resources became scarce or natural predators were introduced. I predict that the short eyestalk population would outcompete their bug-eyed relatives, indicating that any genetic benefit was outweighed by the detriment of the handicap. If they did, that would raise serious questions about how such a trait preference could actually arise in the first place via the Handicap Hypothesis.

Fig. 6. Stalk-eyed fly. Ton Rulkens, “Stalk-Eyed Fly (Diasemopsis) (4561140578),” https://commons.wikimedia.org/wiki/File:Stalk-eyed_fly_(Diasemopsis)_(4561140578).jpg. CC BY-SA 2.0.
The Design Model
If the Handicap Hypothesis is not the right answer, what is going on with these extravagant displays? Why would a female choice for such biologically wasteful and counter-intuitive extravagance exist? Creationists have proposed that these scenarios arise from the creativity of an Artist who was interested in beauty for its own sake and for the purpose of communicating a message to His sentient creatures. Jesus pointed to the extraordinary beauty of a colorful lily as he taught the lesson of God’s care for creation (Luke 12:27). In Job 39 we read God’s own instruction to the patriarch concerning his creation of the ostrich (verses 13–18). God purposely deprived this bird species of the genetic instinct for maternal care so that “she is hardened against her young ones, as though they were not hers.” It would seem logical that God might also have endowed other bird species with sexual preferences that would not maximize survival.
The design model seems to be a better fit for many of the extreme cases of beauty, like that seen in a peacock’s tail, than the naturalistic model. Studies have been equivocal on whether the peahen even shows a preference for more ornate peacock tails (Anderson 1994; Cronin 1991, 118; Takashi et al. 2008; Yorzinski et al. 2013). After all, how can the eye of a peahen appreciate the subtle details of the “eyes” on the peacock’s tail? Darwin himself recognized this difficulty with his theory of sexual selection: “Many will declare that it is utterly incredible that a female bird should be able to appreciate fine shading and exquisite patterns. It is undoubtedly a marvellous fact that she should possess this almost human degree of taste” (Darwin 1888, 349). So while no preference gene has yet been identified, its existence would not be problematic for the creation model like it is in the Darwinian model.
Mutual sexual selection (both the males and females expressing preferences) has also been presented by evolutionists as a common biological phenomenon. This mechanism of mate choice has particularly been employed to explain exaggerated traits seen in both sexes among birds. It was initially proposed to explain the origin of displays in the great crested grebe, Podiceps cristatus (Huxley 1914). In more modern times, this has been applied to the social life of everything from fruit flies (Chenoweth and Blows 2003) to giant pandas (Adilia and Petrova 2016). This requires preference genes to be present in both sexes of those species. But rather than appealing to preference genetics and complicated handicap signaling in both sexes, perhaps the more parsimonious explanation is that God just made that species to be beautiful “And God saw every thing that he had made, and, behold, it was very good. And the evening and the morning were the sixth day.” Genesis 1:31.
Not every population is experiencing a competitive “struggle to the death” for existence at every given moment. So, there is some latitude to have diversity and beauty, even at a cost to maximizing a population’s growth. The design hypothesis is well-articulated by Stuart Burgess:
. . . the Creator may have installed a preference gene as a means of ‘maintaining’ beautiful features. Beauty generally gives a disadvantage in terms of escaping from predators. If a peacock lost its colours due to a gene mutation, it would suddenly find itself more protected from predators. This is an example of where a loss of information could be a great advantage in terms of survival. Therefore, it is conceivable that the Creator would deliberately create preference genes for prominent aesthetic features such as colour. (Burgess 2001, 99)
However, maintenance of “exaggerated” traits poses a challenge within the design model as well.
A Challenge within the Design Model
Creationists have pointed to problems in the secular model involving great eons and random mutations developing and maintaining a sexual selection paradigm (Burgess 2001). Certainly prolonged periods of stability (little differentiation in the genetic quality of the males) should have led to a random loss-of-function mutation in the females, such that preference genes were eliminated. With little difference in males, eliminating the pressure for a handicap would have meant immediate advantage over one’s peers. So how come these handicaps persisted? This issue must be addressed within the young-earth creationist model.
Natural selection, at least as a micro-evolutionary mechanism, is accepted by most creationists. It is typically viewed as a conservative force to maintain a healthy population and to allow morphing within pre-determined limits to adapt to a changing environment. If a given attribute (like cryptic coloration) is an option provided by predesigned genetic information and it offers a sufficient survival advantage, such that it rises above the background noise of happenchance survival events and competing attributes, then it can readily become fixed in the population. Since less ostentatious alleles of many of the attributes we have been discussing would seem to be easily achieved through basic mutations, why do costly and extravagantly beautiful traits still exist millennia after the original creation?
Why, for example, has not a cryptic coloration option for males come into play for the common crimson C. cardinalis (Fig. 7)? Numerous arctic animal varieties, like the polar bear, ptarmigans, Siberian hamster, and arctic fox, enjoy the adaptation of white coloration. Cardinalis is faced with numerous raptor predators like hawks, eagles, and owls. It would seem that cryptic coloration, already genetically expressed in the females, would add significant survival advantage in the male. Assuming God intended to have brilliant birds like cardinals around for the long-term, how might He have ensured that traits that seems to run counter to the bird’s survival persist? Burgess 2001 suggested that the Creator installed preference genes to help stabilize these beautiful traits within the population. But we have already noted that many of the scenarios empirically tested do not confirm the predictions of sexual selection theory and so prominent evolutionists are abandoning it (Roughgarden and Akçay 2010). Even in the classic case of the peacock, something besides the “choosy females” seems to be going on. So, while female preference genes may help lock in some of the colorful male traits over time, there probably is a further explanation.

Fig. 7. Cardinalis cardinalis. https://fthmb.tqn.com/oUooaJOg3rSAR3MngS3YnMpxvQk=/1500x1000/filters:fill(auto,1)/cardinal-snow-58a6cb053df78c345b4454f9.jpg.
An Alternative Hypothesis
If preference genes are a significant biological feature, it seems that they are a variable one. Reduction of female “choosiness” has been observed under certain biological pressures. For example, studies on guppies and swordtails showed that sympatry with similarly ornamented species resulted in the females having reduced preference for ornamentation. Studies among male finches and phrynosomatid lizards demonstrated that pressure to reduce aggressive interactions and territoriality resulted in a reduction or even the loss of male ornaments (Naish 2012). Moreover, female mating preferences were observed to morph in Atlantic mollies, Poecilia Mexicana, when in the presence of predators. “In dichotomous choice tests predator-naïve (lab-reared) females altered their initial preference for larger males in the presence of the cichlid Cichlasoma salvini, a natural predator of P. Mexicana, and preferred small males instead. This effect was considerably weaker when females were confronted visually with the non-piscivorous cichlid Vieja bifasciata or the introduced non-piscivorous Nile tilapia (Oreochromis niloticus)” (Bierbach et al. 2011).
These cases demonstrate built-in flexibility in the female preference trait (and probably also indicate multiple genes are involved). They have the hallmarks of designed systems, where competing goals are weighed and prioritized by sophisticated logic routines. In the trade-off between survival and beauty, survival prevails. Perhaps some of the drab bird species of today were once more beautiful in their natural condition. Selective breeding has revealed the genetic capacity for brilliant coloration in various species. For example, brown carp were turned into gaudy koi over the centuries by Japanese breeders. These complex genetic scenarios lead me to postulate that there are also sophisticated genetic designs involved in those cases where extravagant beauty displays have been maintained and there is no evidence of female preference in place to stabilize them over the millennia.
Wilkinson and Reillo’s work highlighted complex genetic linkage between the male traits and female choices. I would go one step further in postulating gene correlations and suggest that we might be dealing with pleiotropic situations. It could be that some critical biological traits, which are fairly widespread in the animal kingdom, are genetically linked to the male genes for the exaggerated trait. To eliminate the genes for the “handicap” ornament might imperil something important for survival. This hypothesis potentially harmonizes with John Maynard Smith’s Index formulation (see above). In some cases, females help maintain ornamentation via “choosy” genes and in others, males themselves maintain the genetics for ornamentation for survival reasons. By embedding the requirement for the ornament, the degree of brilliance exhibited could convey male genetic quality to suitors. The end result is the maintenance of a more beautiful (and perhaps healthier) population that fulfills the Designer’s original purpose.
As genetic mapping tools continue to advance, this pleiotropic genetic hypothesis could be tested. The genes for the “handicap” ornaments need to be identified first. Then they could be “knocked out” via an editing tool like CRISPR-Cas9. Observations of the progeny could not only confirm that the beauty trait was gone, but it should be apparent if some other important trait was knocked out as well. If this proved to be the case, I believe even those who are seeking only naturalistic explanations would find this alternative hypothesis to be superior to the Handicap Hypothesis.
Conclusion
But ask now the beasts, and they shall teach thee; and the fowls of the air, and they shall tell thee: (Job 12:7, KJV)
The Handicap Hypothesis continues to face significant criticism, even from within the evolutionary community and there are serious theoretical challenges regarding the origin of a biological handicap. Falsifying the handicap hypothesis would seem to be experimentally possible and should be pursued. Rather than assuming that an extravagant trait existed in a species because it added direct survival benefit in a particular environment or communicated something important within a sexual selection paradigm, biologists should also be asking if perhaps there was a design purpose behind it. Might it be beauty “baked-in” by the Creator to carry a message? Might it be maintained by “piggybacking” upon other critical traits? Unlike so many of the “just so” selection stories that abound in biology circles, this pleiotropic genetic hypothesis could become a productive, testable explanatory tool.
References
Adilia, A. S., and E. Petrova. 2016. “Mutual Sexual Selection Helps Evolution, Scientists Say.” http://blogs.elenasmodels.com/en/mutual-sexual-selection-helps-evolution/.
Andersson, M. 1982. “Female Choice Selects for Extreme Tail Length in a Widowbird.” Nature 299 (5886): 818–820.
Andersson, M. B. 1994. Sexual Selection. Princeton, New Jersey: Princeton University Press.
Bierbach, D., M. Schulte, N. Herrmann, M. Tobler, S. Stadler, C. T. Jung, B. Kunkel, et al. 2011. “Predator-Induced Changes of Female Mating Preferences: Innate and Experiential Effects.” BMC Evolutionary Biology 11: 190.
Burgess, S. 2001. “The Beauty of the Peacock Tail and the Problems With the Theory of Sexual Selection.” TJ 15 (2): 94–102.
Cain, M. L., W. D. Bowman, and S. D. Hacker. 2014. Ecology. Sunderland, Massachusetts: Sinauer Associates.
Chenoweth, S. F., and M. W. Blows. 2003. “Signal Trait Sexual Dimorphism and Mutual Sexual Selection in Drosophila serrata.” Evolution 57 (10): 2326–2334.
Cronin, H. 1991. The Ant and the Peacock. Cambridge, United Kingdom: Cambridge University Press.
Curry, J. R. 2008. Children of God: Children of Earth. Bloomington, Indiana: AuthorHouse.
Darwin, C. 1858. On the Origin of Species. New York, New York: Penguin Books.
Darwin, C. 1888. The Descent of Man, and Selection in Relation to Sex. London, United Kingdom: John Murray.
Fisher, R. A. 1930. The Genetical Theory of Natural Selection: A Complete Variorum Edition. Oxford, United Kingdom: Oxford University Press.
Grafen, A. 1990. “Biological Signals as Handicaps.” Journal of Theoretical Biology 144 (4): 517–546.
Grose, J. 2011. “Modelling and the Fall and Rise of the Handicap Principle.” Biology & Philosophy 26 (5): 677–696.
Huttegger, S. M., J. P. Bruner, and K. J. S. Zollman. 2015. “The Handicap Principle Is an Artifact.” Philosophy of Science. 82 (5): 997–1009.
Huxley, J. 1914. “The Courtship Habits of the Great Crested Grebe (Podiceps cristatus); With an Addition to the Theory of Sexual Selection.” Proceedings of the Zoological Society of London 35: 491–562.
Johnstone, R. A. 1995. “Sexual Selection, Honest Advertisement and the Handicap Principle: Reviewing the Evidence.” Biological Reviews 70 (1): 1–65.
Kane, P., and K. J. S. Zollman. 2015. “An Evolutionary Comparison of the Handicap Principle and Hybrid Equilibrium Theories of Signaling.” PloS ONE 10 (9): e0137271.
Kirkpatrick, M. 1986. “The Handicap Mechanism of Sexual Selection Does Not Work.” The American Naturalist 127 (2): 222–240.
Lachmann M., S. Számadó, and C. T. Bergstrom. 2001. “Cost and Conflict in Animal Signals and Human Language.” PNAS 98 (23): 13189–13194.
Naish, D. 2012. “Did Dinosaurs and Pterosaurs Practise Mutual Sexual Selection?” Tetrapod Zoology Blog. Scientific American. https://blogs.scientificamerican.com/tetrapod-zoology/mutual-sexual-selection-dinosaurs-and-pterosaurs/.
Roughgarden, J., and E. Akçay. 2010. “Do We Need a Sexual Selection 2.0?” Animal Behaviour 79 (3): e1–e4.
Roughgarden, J., M. A. Oishi, and E. Akçay. 2006. “Reproductive Social Behavior: Cooperative Games to Replace Sexual Selection.” Science 311 (5763): 965–969.
Smith, J. M. 1976. “Sexual Selection and the Handicap Principle.” Journal of Theoretical Biology 57 (1): 239–242.
Smith, J. M. 1985. “Sexual Selection, Handicaps and True Fitness.” Journal of Theoretical Biology 115 (1): 1–8.
Takahashi, M., H. Arita, M. Hiraiwa-Hasegawa, and T. Hasegawa. 2008. “Peahens do not Prefer Peacocks With More Elaborate Trains.” Animal Behaviour 75 (4): 1209–1219.
Wilkinson, G. S., and P. R. Reillo. 1994. “Female Choice Response to Artificial Selection on an Exaggerated Male Trait in a Stalk-Eyed Fly.” Proceedings of the Royal Society B 255 (1342): 1–6.
Yorzinski, J. L., G. L. Patricelli, J. S. Babcock, J. M. Pearson, and M. L. Platt. 2013. “Through Their Eyes: Selective Attention in Peahens During Courtship,” Journal of Experimental Biology 216 (16): 3035–3046.
Zahavi, A., 1975. “Mate Selection—A Selection for a Handicap.” Journal of Theoretical Biology 53 (1): 205–214.
Zahavi, A., and Zahavi, A. 1997. The Handicap Principle: A Missing Piece of Darwin’s Puzzle. Oxford, United Kingdom: Oxford University Press.
Zollman, K. J. S. 2013. “Finding Alternatives to Handicap Theory.” Biological Theory 8 (2): 127–132.