The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
Parasitology is the study of the symbiotic interaction between two different species of organisms in which one (parasite) lives in or on the other (host) and is metabolically dependent upon it. This large field includes the study of community and microbial ecology. Man and animals provide the ecological niches parasites inhabit and other organisms (for example, symbionts) they encounter. There is a need for parasites such as Entamoeba histolytica to be addressed from a biblical perspective that may include their original symbiotic or mutualistic association in man. E. histolytica is a protozoan parasite of the family Entamoebae that is found throughout the world killing approximately 100,000 people per year. Eight amoebas (Endolimax nana, Entamoeba coli, E. histolytica, E. dispar, E. hartmanni, Iodamoeba bütschlii, E. moshkovskii and E. polecki) reside in the human intestinal lumen. In the pre-fallen world there was true ecological harmony with an intimate association of individuals of different species (symbiosis). Creation microbiologists are currently investigating the role of single-celled eukaryotic creatures in the organosubstrate model. While the controlling factors that direct invasiveness of E. histolytica are not well understood, the progression from originally free-living single-celled eukaryotes (neutral or beneficial) toward a pathogenic condition after the Fall could have occurred through a number of mechanisms leading to a parasitic condition.
Keywords: microbial ecology, organosubstrate, parasitism, creation
Introduction
Allaby (1992) defined parasitology as
the study of small organisms (parasites) living on or in other organisms (hosts), regardless of whether the effect on the hosts is beneficial, neutral or harmful.
The wide field of parasitology is a study of community ecology and association patterns as it applies to host, parasite, and their environment (Caron et al. 2009; Patterson and Banks 2001; Roberts and Janovy 2009). Meerovitch (1982) stated,
The host-parasite relationship in amoebiasis is among the most complex ones in parasitic infections in general.
Amoebas are complex, single-celled eukaryotic protists that utilize pseudopodia for locomotion. Entamoeba histolytica is the causative agent of amoebiasis that occasionally results in death. Given the ubiquity of amoebas, one might ask how they were related to man and animal in a pre-Fallen world. Such a hypothesis would aid in describing the origin of parasitism (that is, amoebiasis) after the Fall.
Scripture teaches that prior to the Fall, everything was very good (Genesis 1:31). There was true ecological harmony with an intimate and extended association of individuals of different species (symbiosis). In fact, amoebas seemed to be designed to form symbiotic communities. Several species of harmless amoebas living in the human intestine (for example, E. hartmanni) today may reflect the pre-Fall condition. Such undisruptive association could have been mutualistic, just as Escherichia coli is within the human host today. After the Fall God cursed all of creation, for example the ground was cursed with thorns and thistles (Genesis 3). In addition, the removal of some of God’s sustaining power could have resulted in a conversion via displacement and modification leading from neutral or beneficial protozoa to pathogenic species (Francis 2009). A synthesis of organosubstrate model (and possibly serial endosymbiosis) with single-celled ecology may help in our understanding of free-living amoeba (or originally mutualistic amoeba) and their transition to parasitism. Indeed, the hypothesis (Bergman 1999) of the creation model of microbiology includes bacteria, fungi, and protozoans that may have been beneficial, commensal (symbiotic association between two species in which one benefits, the commensal, and the other is not affected), or mutualistic in their interaction with people and animals.
Classification of the Amoeba
Roberts and Janovy (2009) stated scientists have been occupied for over two centuries regarding the “monumental” task of classifying eukaryotic microorganisms. Amoeba are defined as single-celled eukaryotic protists. In decades past amoebas were classified within the phylum Protozoa. However, the term protozoa is colloquial and used today as a common noun with no taxonomic significance. There is much uncertainty regarding utility, classification schemes, and the alleged common ancestry of the amoeba (Roberts and Janovy 2009). Currently the amoeboid protozoa belong to a major group called the Amoeboza. In 2006 Adl and 27 others attempted to classify this large group of eukaryotes (Adl et al. 2006). Regardless, the taxonomy of the amoebas remains “extremely unsettled” (Roberts and Janovy 2009). Evolutionists do not know the origin of amoebas, parasitic or benign, and numerous amoebic genes lack homologs in other systems (MacFarlane and Singh 2007). Evolutionists do place E. histolytica on a branch of the eukaryotic diagram based on rRNA phylogenies (Clark and Roger 1995), but they can say only that all organisms “probably” evolved from a common, single-celled progenitor (Lodish et al. 2008, p. 4). Current molecular data used, in the case to answer classification questions of single-celled creatures, has been contrary to older classification systems. The Archamoebae (ancient or primitive amoeba) are a very diverse collection, and for that reason some evolutionists “doubt the phylogenetic unity of the group” (Keeling 1998, p. 89). Creationists maintain diverse groups of microorganisms were created after their kind to be ubiquitous, abundant, and form symbiotic microbial communities (Francis 2003).
Ecology of Entamoeba histolytica
Amoebas may be parasitic (for example, E. histolytica), commensal, or free living. Eight amoebas (Endolimax nana, Entamoeba coli, E. histolytica, E. dispar, E. hartmanni, E. moshkovskii, E. polecki, and Iodamoeba bütschlii) reside in the human intestinal lumen (Diamond and Clark 1993; Tanyuksel and Petri 2003) as evident commensals, deriving a niche and sustenance. That these amoebas contribute to a mutualistic relationship cannot be determined as no benefit can be seen in the host. There are two exceptions (I. bütschlii, E. polecki) that have been suspected of mild pathogenesis (Roberts and Janovy 2009).
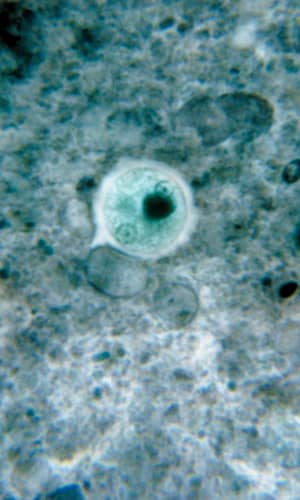
Entamoeba histolytica is a tissue-lysing luminal protozoan parasite of the family Entamoebae that is found throughout the world (McLaughlin and Aley 1985). Its life cycle includes two stages, the infectious (cyst) stage and the pathogenic trophozoite stage. E. histolytica kills about 100,000 people per year and infects about 50,000,000 more (Roberts and Janovy 2009). About 85% of people infected with this parasite are healthy carriers (Noble et al 1989). E. histolytica is the causative agent of what has been termed the “dirty hands disease.” For example, infected food handlers with poor sanitation habits can easily pass cysts on to others. Contaminated drinking water and mechanical vectors such as cockroaches and flies are also a potential source of infection. A significant portion of the homosexual population is infected through fecal/oral contamination (Noble et al 1989) where it can reach epidemic levels.
After being eaten by a new host, cysts are transported to the small and large intestine. The cysts pass through the stomach unscathed and display no activity while exposed to the low pH. But upon reaching the higher pH of the small intestine, metacysts start to move within the walls of the cyst which weaken and open. Excystation (emergence) occurs and the quadrinucleate amoebas divide into amoebulas (daughter cells of encysted amoeba) and move rapidly downstream. The amoebas reside in the lower portion of the small intestine and the lumen of the large intestine. The trophozoites reproduce via binary fission within the crypts of the large intestine mucosa (where pH is more stable), apparently feeding on mucous secretions and polysaccharides and undergoing metabolic interaction with proteobacteria. Although intestinal bacteria evidently are important in function and growth of amoebas, it is not well understood and biologists continue to investigate this complex relationship. In terms of the organosubstrate model, the bacteria perhaps protected the host while the amoebas provided an as-yet-unidentified benefit. Indeed, evidence accumulates regarding immune system benefits of good bacteria (Mazmanian 2009). Under the “right” conditions (for example, pathogenesis of the amoeba, immunity status and nutrition of the host and the bacterial flora) the trophozoites may initiate tissue invasion when they break down mucosal cells and absorb the products. The amoebas at this point no longer need bacterial presence to satisfy their nutritional demands. This parasite has a number of mechanisms to invade host tissues that includes contact-dependent cytolytic mechanisms and biochemical mechanisms such as secreted hydrolytic enzymes (proteinases, phosphatases, and glycosidases).
Those individuals new to endemic areas may suffer from amoebic infection due to differences in their bacterial flora. The amoebas may become invasive, producing ulcers in the intestinal wall, reaching the submucosa and blood vessels, and thence transported by the blood stream. After a period of time, amoebas take on a round shape, form a cyst wall, and become a glycogen-rich precyst. Mature cysts have a robust hyaline cyst wall in the large intestine and are liberated from the host in great numbers. A cystproducing individual may pass up to 45 million cysts per day while being asymptomatic or only mildly afflicted.
Infections are 40% of the population in tropical areas. Approximately 90% of people who become infected with this parasite are asymptomatically colonized (Guerrant 1986). Clinical manifestations of E. histolytica infection are amoebic liver abscess and amoebic colitis (Elsdon-Dew 1964; Stanley and Reed 2001), but, other than the above discussion, the factors that control the invasiveness of E. histolytica are not well understood (Tanyuksel and Petri 2003) and its modus operandi is largely unknown (MacFarlane and Singh 2007). What is known is the relationship between this amoeba and the host is doubtless one of the most complex in terms of general parasitic infections (Meerovitch 1982; Noble 1989).
Parasites are designed to adapt to different micro- and macroenvironments in the host and between hosts. E. histolytica is mainly a coelozoic microparasite that lives with a number of other (nonpathogenic) amoebas in what is probably a commensalistic environment.
The ecology of nonpathogenic amoeba prior to the Fall would perhaps be similar to that of many present noninfectious amoebas. There is much work to be done, however, as community ecology (and specifically as it applies to parasitic protozoa in this paper) has yet to experience serious research (Little et al. 2008). Indeed, when it comes to the microbial community on and within people, biologists are finding ecorelationships (ecological webs) are more complex with a population of organisms being commensal under certain conditions and beneficial under others (Little et al. 2008). Such ecological webs with their multifunctional components have been likened to complex engineered systems (Kitano 2002). Kitano’s logical comparison of these webs to overtly designed computational systems has not been overlooked by this writer.
Amoebas in Organosubstrate Model: A Proposed Creation Explanation
Evolutionists have recently admitted, “True, the earliest microbial stories are laced with conjecture and may not be altogether coherent” (Fox 2009, p. 210). Conversely, the organosubstrate model (Francis 2003) states microbes such as amoebas could be seen as part of a large, sophisticated multitaxon organism with amazing and potent life-supporting properties. Purdom and Francis (2008) state,
[microbes] . . . serve as intermediaries between living things and the physical world around them. Just as we have organelles (tiny organs) inside our cells, microbes might be thought of as extracellular organelles that help living things interact with their environment.
For instance, it is predicted that microbes reside on every living creature, where they have been shown to play long-term beneficial and life-sustaining roles, and thus it appears that microbes could have been created as part(s) of each of the original baramins (Purdom 2008). Free living amoebas are found in the designed ecosystems of our world from pole to pole (Brown, Cursons, and Keys.1982), from some deep aquifers systems (Novarino et al. 1997) to caves (Gittleson and Hoover 1970), and from mines (Johnson and Rang 1993) to seaweed fronds (Armstrong, Rogerson, and Leafley 2000). Amoebas are particularly abundant in soils where they and other single-celled eukaryotes limit bacterial population sizes, affecting the soil’s microbial composition (Murase, Noll, and Frenzel 2006). Surprisingly, cyst-free amoebas have even been found in the atmosphere (Kingston and Warhurst 1969; Rivera et al. 1987). They are also found in freshwater and marine waters and at their greatest numbers in biofilms (Barbeau and Buhler 2001). The wide-ranging nature of the amoebas clearly fits with the organosubstrate prediction of their being designed with survival mechanisms and mechanisms of proficient reproduction (Francis 2003). Biblical creation science would possibly combine the organosubstrate model and modified serial endosymbiosis [that states organelles originated as unique prokaryotic organisms that were taken inside a cell as endosymbionts. Mitochondria developed from proteobacteria (perhaps Rickettsiales) and chloroplasts from cyanobacteria] (Margulis and Sagan 2001). Such an amalgamation of the two models may further help in our understanding of free-living and parasitic protozoa such as E. histolytica (and possibly helminths).
Scripture teaches that prior to the Fall, everything was very good (Genesis 1:31). There was true ecological harmony with an intimate and extended association of individuals of different species (symbiosis). In fact, amoebas seemed to be designed to form symbiotic communities. Perhaps a glimmer of this pre-Fall condition can be seen today with several species of harmless amoebas living in the human intestine (for example, E. hartmanni). Such undisruptive association could have been mutualistic, just as E. coli is within the human host today. After the Fall God cursed the Earth, for example the ground was cursed with thorns and thistles (Genesis 3). Creationists maintain that in regard to the biblical model of creation, corruption and curse, there has been a progression of free-living animals destined to their parasitic mode that proceeds from protagonistic, to benign (or phoretic), to an antagonistic or parasitic relationship (Blanc 1992; Little et al. 2008). The period of time of this conversion process is not known, but it could have occurred in “practically a one-step transformation” (Noble, et al, p. 517).
How did this progression occur? The field of bacteriology points to genetic shuffling, genomic decay and/or corruption—
Many infectious diseases can be traced back to the decay and corruption of the original created design of microorganisms as a result of the Fall. Corruption literally means to destroy (from the Latin corruptus). The origin of pathogenic (disease-causing) bacteria such as Y. pestis is complex and multifaceted, and may be explained by a combination of genes that were lost, added and moved. The story of Yersinia’s degeneration into the plague pathogen may serve as a model of “fast” genomic decay and corruption (Gillen and Sherwin 2006, p. 7).
Recent bacteriologic research shows a blurring between commensals and pathogens (Marshall, Ochieng, and Levy 2009). Could such blurring also apply to amoebas? E. histolytica could very well have been a commensal such as the other seven protozoa found in the human gut. Was the purpose(s) of E. histolytica to control bacterial numbers in this environment? Or perhaps it had a more mutualistic relationship? Microbiologists are now finding that a number of commensals are in fact critical factors involving, for example, nutrient absorption and development of the immune system (Hooper, Midtvedt, and Gordon 2002; Mazmanian, 2009; Rakoff-Nahoum et al. 2004). After the Fall, mutations of the protein coding sequences (regulatory regions) or horizontal gene transfer (Little et al. 2008), possibly led to “fast” genomic decay and corruption, resulting in a parasitic condition.
After the Fall, some of the amoeba originally linked between macro-organisms and the inert physical environment (Francis 2003), took on a pathological role. But why trophozoites invade (that is, become pathogenic) is still largely unanswered (Noble 1989). It is known that bacteria are able to adapt to some extent to unexploited ecological niches and become pathogenic via horizontal gene transfer (Osborne et al. 2008). Interestingly, recent research has shown evidence of lateral gene transfer of bacterial genes into the genome of E. histolytica (Loftus et al. 2005). It is thought such a transmission would increase the metabolic range of this parasite. Perhaps this was the route taken, in part, regarding the pathogenicity of E. histolytica as well as other protozoa and bacteria (Gillen and Sherwin 2006; Osborne et al. 2008). There is an amoeba identical to E. histolytica. E. moshkovskii and E. histolytica have identical morphology, but the former is not a symbiont and lives in sewage. Creation scientists suggest, as a result of the Curse, E. histolytica became more aggressive, possibly overcame the barrier effect established by resident microflora (Guarner and Malagelada 2003), and became a pathogen.
Future creation parasitology research should address why E. histolytica has filopodia and membrane lectins. The parasite also has cysteine proteases and proteolytic enzymes (neutral proteases and collagenase) designed to facilitate tissue invasion (Tanyuksel and Petri 2003). The metabolism and biochemistry of this protozoan parasite has been reviewed by McLaughlin and Aley (1985). It is currently not known the role of these enzymes prior to the Fall. Perhaps they were used to metabolize prokaryotes within the host GI tract. E. histolytica today is found with food vacuoles in the cytoplasm containing bacteria. (Roberts and Janovy 2009)
Conclusion
The case can clearly be made for creation as we see overt design features of parasites and their symbiotic or mutualistic association in man. However, explaining or suggesting purpose and function of parasites (that is, E. histolytica) before the Fall, and the morphological and physiological changes they evidently underwent, requires extensive reflection and, when possible, research. The original function of E. histolytica can only be theorized. The complex physiology and ecology of this protozoan (for example, immune system evasion and bacterial flora interaction) indicates as yet unexplained pre-Fall/Curse functions. Researchers have discovered that a number of commensals are in fact critical factors. A synthesis of organosubstrate model with single-celled eukaryotes may help in our understanding of free-living amoeba (or originally mutualistic amoeba) and their transition to parasitism.
Questions for Further Research
Where do single-celled eukaryotes fit in regarding the nutrient (bacteria) and decay (fungi) cycles in the organosubstrate theory?
Why was Entamoeba histolytica created? Was its original function to be an “intermediary between the inorganic world and macro-organisms”?
If E. histolytica lived mutualistically within the human host prior to the Fall, what did it produce? What were its benefits?
What was the role of obligate parasites in the pre-Fall world?
When and how did amoebas become invasive?
E. histolytica has a complex cyst stage—E. gingivalis does not. Why?
Since the Fall, have parasites (E. histolytica) achieved an optimal virulence maximizing their numbers?
References
Adl, S.,et al. 2006. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. Journal of Eukaryotic Microbiology 52:399–451.
Allaby, M. 1992. The concise Oxford dictionary of zoology, p. 340. Oxford University Press.
Armstrong, E., A. Rogerson, and J. W. Leafley. 2000. The abundance of heterotrophic protists associated with intertidal seaweeds. East Coast Shelf Science 50:415–424.
Barbeau, J. and T. Buhler. 2001. Biofilms augment the number of free-living amoebae in dental unit waterlines. Microbiology 152:753–760.
Bergman, J. 1999. Did God make pathogenic viruses? CEN Technical Journal 13, no. 1:115–125.
Blanc, D. 1992. Determination of taxonomic status of pathogenic and nonpathogenic Entamoeba histolytica zymodemes using isozyme analysis. Journal of Protozoology 39:471–479.
Brown, T. J., R. T. M. Cursons, and E. A. Keys. 1982. Amoebae from Antarctic soil and water. Applied Environmental Microbiology 44:491–493.
Caron, D. et al. 2009. Microbial eukaryote diversity and biogeography. Microbe 4:71–77.
Clark, C., and A. Roger. 1995. Direct evidence for secondary loss of mitochondria in Entamoeba histolytica. Proceedings of the National Academy of Science 92:6518–6521.
Diamond, L., and C. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. Journal of Eukaryotic Microbiology 40:340–344.
Elsdon-Dew, R. 1964. Amoebiasis. Experimental Parasitology 15:87–96.
Fox, J. 2009. When earth was primordial soup. Microbe 4:210–212.
Francis, J. 2003. The organosubstrate of life. In Proceedings of the Fifth International Conference on Creationism, ed. R. L. Ivey, pp. 433–444. Pittsburgh, Pennsylvania: Creation Science Fellowship.
Francis, J. 2009. Good designs gone bad. Answers 4, no. 3: 32–35.
Gillen, A., and F. J. Sherwin. 2006. The origin of bubonic plague. Journal of Creation 20, no. 1:7–8.
Gittleson, S. M., and R. C. Hoover. 1970. Protozoa of underground water in caves. Annual Spelology 25:91–106.
Guarner, F., and J. Malagelada. 2003. Gut flora in health and disease. Lancet 361:512–519.
Guerrant, R. L. 1986. Amebiasis: introduction, current status, and research questions. Review of Infectious Disease 8:218–227.
Hooper, L., T. Midtvedt, and J. Gordon. 2002. How hostmicrobial interactions shape the nutrient environment of the mammalian intestine. Annual Review of Nutrition 22:283–307.
Johnson, D. B., and L. Rang. 1993. Effects of acidophilic protozoa on populations of metal-mobilizing bacteria during the leaching of pyritic coal. Journal of General Microbiology 139:1417–1423.
Keeling, P. J. 1998. A kingdom’s progress: Archezoa and the origin of eukaryotes. BioEssays 20:87–95.
Kingston, D., and D. C. Warhurst. 1969. Isolation of amoebae from the air. Journal of Medical Microbiology 2:27–36.
Kitano, H. 2002. Computational systems biology. Nature 420:206–210.
Little, A., C. J. Robinson, S. B. Peterson, K. F. Raffa, and J. Handelsman. 2008. Rules of engagement: Interspecies interactions that regulate microbial communities. Annual Review of Microbiology 62:373–401.
Lodish, H., A. Berk, C. A. Kaiser, M. Krieger, M. P. Scott, A. Bretscher, H. Ploegh, and P. Matsudaira. 2008. Molecular cell biology, 6th ed. New York: W. H. Freeman & Company, New York.
Loftus, B., I. Anderson, R. Davies, U. C. M. Alsmark, J. Samuelson, P. Amedeo, P. Roncaglia, M. Berriman, R. P. Hirt, B. J. Mann, T. Nozaki, B. Suh, M. Pop, M. Duchene, J. Ackers, and E. Tannich. 2005. The genome of the protist parasite Entamoeba histolytica. Nature 433:865–868.
Marshall, B., D. Ochieng, and S. Levy. 2009. Commensals: underappreciated reservoir of antibiotic resistance. Microbe 4:231–238.
MacFarlane, R., and U. Singh. 2007. Identification of an Entamoeba histolytica serine-, threonine-, and isoleucinerich protein with roles in adhesion and cytotoxicity. Eukaryotic Cell 6:2139–2146.
McLaughlin, J., and S. Aley. 1985. The biochemistry and functional morphology of the Entamoeba. Journal of Protozoology 32:221–240.
Mazmanian, S. 2009. The microbial health factor. New Scientist 23:34.
Meerovitch, E. 1982. The jigsaw puzzle of host-parasite relationships in amoebiasis begins to take shape. In Aspects of parasitology, ed. E. Meerovitch, pp. 263–278. Montreal: Institute of Parasitology.
Murase, J., M. Noll, and P. Frenzel. 2006. Impact of protists on the activity and structure of the bacterial community in a rice field soil. Applied Environmental Microbiology 72:5436–5444.
Noble, E., G. A. Noble, G. A. Schad, and A. J. MacInnes. 1989. Parasitology. Philadelphia: Lea & Febiger.
Novarino, G., W. A. Butler, G. Lambourne, R. W. Harvey, R. A. Mosse, and B. Teltsch. 1997. Protistan communities in aquifers: A review. FEMS Microbiology Reviews 20: 261–275.
Osborne, S. et al. 2008. Pathogenic adaptation of intracellular
bacteria by rewiring a cis-regulatory input function.
Proceedings of the National Academy of Sciences (early
edition)
http://www.pnas.org/content/early/2009/02/19/0811669106.abstract
Patterson, A., and J. Banks. 2001. Analytical approaches to measuring cospeciation of hosts and parasites: Through a glass darkly. International Journal of Parasitology 31:1012–1022.
Purdom, G. and J. W. Francis (eds.) 2008. Proceedings of the Microbe Forum, June 2007. Answers Research Journal 1:1–6.
Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241.
Rivera, A., G. Roy-Ocotla, I. Rosas, E. Ramirez, P. Bonilla, and F. Lares. 1987. Amoebae isolated from the atmosphere of Mexico City and environs. Environmental Research 42:149–154.
Roberts, L., and J. Janovy. 2009. Foundations of parasitology. New York: McGraw Hill.
Margulis, L. and D. Sagan. 2001. Marvellous microbes. Resurgence 206:10–12.
Stanley, S. L. and S. Reed. 2001. Microbes and microbial toxins: Paradigms for microbial-mucosal interactions VI. Entamoeba histolytica: parasite-host interactions. American Journal of Physiology Gastrointestinal Liver Physiology 280:G1049–1054.
Tanyuksel, M., and W. Petri. 2003. Laboratory diagnosis of amebiasis. Clinical Microbiology Review 16, no. 7:13–729.