The views expressed in this paper are those of the writer(s) and are not necessarily those of the ARJ Editor or Answers in Genesis.
Abstract
A discussion on a previous ARJ paper in regard to Australopithecus sediba and its classification.
Todd Wood (2010) raises some provocative questions for creationists about the nature of man. In particular he poses the question “what precisely is human (that is, descendants of Adam and Eve)?” and seems to imply that this problem could not be adequately addressed until the recent advent of cladistics and baraminology. But King David addressed the issue of “what is man?” nearly 3,000 years ago in Psalm 8:4–7:
4 What is man that You are mindful of him, And the son of man that You visit him? 5 For You have made him a little lower than the angels, And You have crowned him with glory and honor. 6 You have made him to have dominion over the works of Your hands; You have put all things under his feet, 7 All sheep and oxen— Even the beasts of the field,
And in his letter to the Corinthians, the apostle Paul declared:
All flesh is not the same flesh, but there is one kind of flesh of men, another flesh of animals, another of fish, and another of birds. (1 Corinthians 15:39)
And finally in the Acts of the Apostles, Luke declared:
And He has made from one blood every nation of men to dwell on all the face of the earth, (Acts 17:26)
Thus according to Scripture, man is a single/specially created kind (min) made in the image of God and is both superior and distinct from all animals. Moreover, all humans are of “one blood” being direct descendants of the first human pair, Adam and Eve. This Scriptural truth is consistent with the fact that today geneticists and physical anthropologists recognize only one living human species (Homo sapiens). Living humans are so similar, that even the once-accepted idea of different races of humans has now been rejected.
It is only in the fossil record where there has been an effort (mostly on the part of evolutionists) to distinguish several different species of Homo. Some Homo specimens like Homo neanderthalensis, are numerous, well-preserved, and clearly human, while others like Homo habilis are fragmentary fossils of questionable relationship to man.
Surprisingly, Wood concludes that the recently discovered ape-like fossil, Australopithecus sediba (which, contrary to the opinion of its discoverer, he prefers to call “Homo” sediba), is in fact human. Like other creationists who have commented on Australopithecus sediba (Line 2010; Thomas 2010 a, b), I do not agree that it is human, and neither do most evolutionists. Paleontologist Tim White (University of California, Berkeley), for example, said that “given its late age and Australopithecus-grade anatomy, it contributes little to the understanding of the origin of genus Homo” (Balter 2010). Similarly, anthropologist Chris Stringer of the Natural History Museum in London said “putting Australopithecus sediba into Homo would require a major redefinition of that genus” (Balter 2010).
In support of his view, Wood uses cladistics which evolutionists define as
a method of inferring evolutionary ancestry by methodically comparing possible evolutionary relationships between organisms and selecting as most likely the relationships which require the fewest number of evolutionary transformations between character states (EverythingBio).
But in the case of fossils, these assumed “relationships” are usually based on similarities of selected teeth and bones from among those that happen to be preserved. This is further complicated by the fact that these presumed “similarities” are the biased opinions of evolutionists.
In the case of Australopithecus sediba, there are only two published specimens (Berger et al. 2010). One is said to be a male (MH1) and the other a female (MH2). This presents a problem because there is a substantial sexual dimorphism in primate skeletal anatomy. To make matters worse, MH1 is a juvenile and MH2 is an adult. Add to this the problem that MH1 consists only of a nearly complete skull and a partial postcranial skeleton, while MH2 consists of maxillary teeth, a partial mandible and a partial postcranial skeleton.
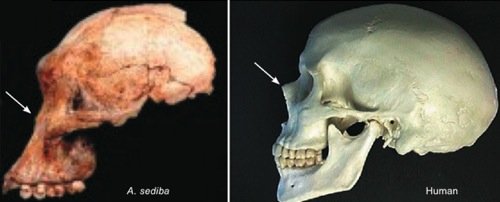
Fig. 1. Comparison of the skulls of Australopithecus sediba and a human. Note the bony protrusion at the human
nose, and the lack thereof on Australopithecus sediba (arrows).
Wood obviously had no access to the Australopithecus sediba fossils which, like hominid fossils in general, are rarely made available by their discoverers for direct analysis by other evolutionists (to say nothing of creationists). Unfortunately, Wood uncritically accepts an evolutionist’s claims of certain anatomical similarities of Australopithecus sediba to humans as hard data, rather than opinion. He then does a statistical analysis of these opinions as though they were objective data. But how similar is Australopithecus sediba to man?
First let us consider just a few of the nontrivial differences between Australopithecus sediba and humans. Australopithecus sediba has a brain measuring between one-third and one-fourth the size of that of a typical human of comparable size (but well within the range of apes). A comparison of the skull of Australopithecus sediba with that of humans (see fig. 1) reveals that the lower face of Australopithecus sediba is sloped like that of apes. And, like apes, the forehead of Australopithecus sediba is flat, making the orbits of the eyes barely visible when viewed from the side. The mandible of Australopithecus sediba bears no close resemblance to that of man (or even a chimpanzee) but rather is more similar to that of a gorilla.
The postcranial skeleton of Australopithecus sediba is also very ape-like. It has a small body with ape-like large-jointed upper and lower limbs. The arms and hands of Australopithecus sediba extend down to the knees, typical of long-armed knuckle walkers. The up-tilted glenoid fossa (shoulder joint) of Australopithecus sediba together with its long curved fingers are typical of the suspensory adapted upper limbs of tree dwelling apes. The feet are described as primitive and similar to other Australopiths. But are these striking differences between Australopithecus sediba and humans outweighed by the similarities?
Paleontologist Tim White said “the characteristics shared by Australopithecus sediba and Homo are few and could be due to normal variation among australopithecines” (Balter 2010). The claimed “Homo-like” features of the Australopithecus sediba pelvis are based on a composite reconstruction of the juvenile MH1 specimen. Any claim of human-like bipedality based on this pelvis is necessarily speculative and subject to the bias of the observer. While most of the claimed similarities of Australopithecus sediba to Homo or humans require knowledge of primate anatomy for their proper evaluation, there is at least one unambiguous claim that can be evaluated by anyone. Australopithecus sediba is claimed to have protruding nasal bones like that of humans (and unlike the flat nasal bones of living apes). Anyone can decide for themselves if this is the case by comparing a side view of the Australopithecus sediba skull with a side view of a human skull (see arrows in fig. 1).
In conclusion, Wood raises several questions that need to be carefully considered by creationists. What exactly is the created “kind” (min) mentioned in the Bible? Is a kind primarily distinguished by a cladistic analysis of selected similarities (while minimizing the differences) or is it primarily determined by the ability to interbreed? What does the creationist mean by animals being “related,” and how does the creationist explain homology, if not reflecting the optimized design of a common Creator?
Have there been several different species of humans in the course of our history, or has man been one unique species from the time of Adam and Eve?
Finally, is man one of a kind, or can humans be taxonomically classified into various species like animals? Specifically, have there been several different species of humans in the course of our history, or has man been one unique species from the time of Adam and Eve? If there were historically several species of humans since Adam and Eve, why do we now only recognize one living species of human kind? May we assume that the first Adam and the last Adam (the incarnate Christ) were the same species? Is this important for Christ’s substitutionary atonement?
These might simply be academic questions were it not for their theological implications. Wood concedes that “human-like animals (or ape-like humans)” are a “theological problem” but insists that “we must develop a new understanding of biological similarity.” But any new understanding should clearly distinguish between observable facts and speculations. Most importantly, the Bible believing creationist will be careful to confine himself to speculations that are consistent with God’s Word.
Anne Habermehl, Independent Scholar, 25 Madison St, Cortland, New York 13045
The purpose of his statistical analysis, as stated by Wood (2010), was to determine whether various fossils were human or not. How successful this method was in achieving its goal is very much in question, since several apes are included in what the analysis declares to be the “human” category.
Will we now accept these obvious apes (such as Australopithecus sediba—and, yes, Wood is totally correct that this sure is a surprise) as human, because somebody’s manipulation of statistics tells us that we should do this? And no matter what fossils this statistical technique dredges up and lumps into the category of humans, are we expected to welcome these beings into our human family with wide open arms? We might well wonder what other fossils will get admitted as humans by the time baraminologists are done.
This paper shows that there is a total faith required of the statistical analysis method itself, the suites of characters chosen, the integrity of these measurements by evolutionists, and the end results. This faith is a troublesome requirement, especially considering the source of those characters. As Jack Cuozzo says,
In all my experience studying craniofacial remains of the Neanderthals and some of the H. erectus specimens, I learned one important lesson. We cannot trust the paleoanthropologist’s reconstructions nor their diagrams or measurements. Given this basic problem, while Wood has done his homework very well, he is too trusting of the characters they choose that he uses in his research. (Cuozzo pers. comm.).
In addition, there are other reasons to be skeptical of the trustworthiness of the characters used in baraminology statistical analysis. Williams (2004), in his review of Understanding the Pattern of Life (Wood and Murray 2003), points out that
some characters are more informative than others when it comes to the history of life, and if the analysis is over-burdened with uninformative characters, it skews the result.
In other words, not all characters are equal when it comes to this kind of analysis. The question is whether we know which characters have greater meaning, and which ones have lesser meaning? I suggest that we do not.
In my paper on Neanderthals, published earlier this year (Habermehl 2010), I point out that we have two possible roads before us with respect to the history of early humans. We can accept the scientific X-ray analysis studies of Cuozzo (1998, passim) and recognize that the long-lived Neanderthals are the only early humans, or we can refuse to accept Cuozzo’s work and have no answers at all on either the Neanderthals or any other claimed humans. By ignoring Cuozzo’s work and its conclusions, and admitting other fossils than Neanderthals as human, Wood is thoroughly confusing the issue of human history. If we creationists really want to develop a harmonized view of our origins, as we claim, we cannot open wide the doors to include all and sundry fossils in our human history.
Lubenow (2004, pp. 246–257) makes a strong argument that the most human practice of all is that of burial. This is a telling point in determining humanness, because humanness is decided, not only by scientific measurement, but also by behavior. No early fossils are known to have buried their dead, except the Neanderthals, as has been pointed out by Cuozzo (pers. comm.).
Historically, we creationists have rather made a laughingstock of ourselves among evolutionists in claiming that we can tell the difference between human and nonhuman fossils (Foley 2008), and this paper does nothing to reverse that situation. The phrase at the end of the abstract, “the significance of human-like australopiths and the ape-like humans” should give creationists pause. This has a distinctly evolutionistic ring to it.
The author states in the abstract,
These results tentatively confirm the common creationist claim that fossil hominids can be divided into human and non-human categories.
Let me point out that we creationists can tell, merely from reading our Bible, that some fossils are human and some are not; we do not need statistical analysis to confirm this.
The enthusiasm of baraminologists for using statistical analysis to determine humanness should perhaps be tempered; indeed, this paper raises very real doubt whether statistical methods should be applied to baraminology at all. There is an a priori assumption inherent in this application of statistics that something useful can be gained from it; the validity of this assumption would appear to be doubtful. In the application of statistical analysis, it is the final results that ultimately tell us whether or not the method has merit as applied; by any statistical standard, this paper is fatally flawed.
David A. DeWitt, Center for Creation Studies, Liberty University, Lynchburg, Virginia 24502
One of the challenges that I have had as a university professor is getting my students to think and reason instead of just taking numbers that come off a calculator or computer. It can be too easy to take an answer that a computer gives as true while failing to recognize obvious problems with that answer. The student goes through wild gyrations to explain why that answer is correct instead of trying to determine what went wrong. There can be hidden assumptions that go into the number that a computer generates, and so it is necessary to interpret it correctly. Computer-produced data must be validated within context and not accepted blindly. To a degree, I fear that this type of phenomena has occurred in Todd Wood’s paper, “Baraminological Analysis Places Homo habilis, Homo rudolfensis, and Australopithecus sediba in the Human Holobaramin” (Wood, 2010).
Wood lays out the purpose for his paper in the introduction. Creationists have disagreed about the status of several fossil hominids and whether these are human, apes, or something else. The disagreement has been exploited by evolutionists to show that these really are ancestors because the creationists can’t tell whether it is human or ape. Wood proposed the use of statistical baraminology to determine which specimens are clearly human, which are not, and where the australopithecines fit.
If the intent was to deflect the criticism of evolutionists, it didn’t work. On the same day Wood’s paper was published, it was criticized by Nick Matzke at Panda’s Thumb (Matzke 2010) as well as by others. No doubt the criticism of “creationist disagreement” on fossil hominids has received significant fuel from Wood’s study, because it is by no means definitive nor the final word on the subject. Specifically, by suggesting that “at least eight species (and possibly three others) within the human holobaramin broadens our understanding of ‘humanity’” (Wood 2010, p. 86) Wood has put himself in disagreement with all of the creationists that he cites.
Not only so, Wood suggests that the post-Babel dispersal of humans was led by Homo habilis and Homo rudolfensis. Since Australopithecus sediba is “unequivocally” within the human holobaramin according to Wood, this should be included in the dispersal although it wasn’t mentioned. He attributes the various hominids to post-Flood diversification and implies that these are all descendents of Adam and Eve through Noah. This is a position that puts him far outside of the mainstream of creationist thinking on the hominid fossils, and one which would require significant and solid support to maintain. Beyond that, even Berger et al. (2010) were not willing to assign Australopithecus sediba to the Homo genus. Therefore, Wood is even in disagreement with the evolutionists who reported the sediba find. This leads to two significant questions. First, do the results from the statistical baraminology make sense in light of the fossil data? Second, if valid, what does that conclusion imply regarding biblical issues?
Australopithecus sediba or Homo sediba?
Berger et al. (2010) provides a strong case for classification of sediba within Australopithecus. First, they state that “The closest morphological comparison for Au. Sediba is Au. africanus.” In fact, Berger et al. place the sediba remains superimposed on an idealized Au. africanus skeleton in a figure in their paper. This is intriguing, because in Wood’s analysis africanus is not correlated with other taxa in one analysis, clearly within Homo in another. Berger et al. place sediba in Australopithecus based on craniodental data including
small cranial capacity, pronounced glabelar region, patent premaxillary suture, moderate canine jugum with canine fossa, small anterior nasal spine, steeply inclined zygomati-coalvelolar crest, high masseter origin, moderate development of the mesial marginal ridge of the maxillary central incisor, and relatively closely spaced premolar and molar cusps.
Berger et al. considered postcranial features including
small body size, relatively long upper limbs with large joint surfaces and the retention of apparently primitive characteristics in the upper and lower limbs.
Other similarities to Australopithecus include
a scapula with a cranially oriented glenoid fossa and a strongly developed axillary border, a prominent conoid tubercle on the clavicle, with a pronounced angular margin; low proximal-to-distal humeral articular proportions; a distal humerus with a marked crest for the brachioradialis muscle, a large and deep olecranon fossa with a septal aperture and a marked trochlear/capitular keel; an ulna with a pronounced flexor carpi ulnaris tubercle; and long robust, and curved manual phalanges that preserve strong attachment sites for flexor digitorum superficialis muscle.”
Some of these traits have functional consequences. Sediba has long upper limbs, which also appear bowed. Curved phalanges with strong muscle attachment sites, and the cranially oriented glenoid fossa mean that sediba would not be using its arms the way essentially all humans do. These represent a suite of ape-like characteristics and allow the arms to be far more weight bearing than humans need for bipedal locomotion. Simply attributing such a group of characteristics as variation does not account for it.
Although features of sediba’s pelvis are derived, the foot was described as primitive and similar to australopithecines with
a flat talar trochlea articular surface with medial and lateral margins with equal radii of curvature, and a short, stout, and medially twisted talar neck with a high horizontal angle and a low neck torsion angle.
The calcaneous was described as primitive. While sediba has some derived characters that make it similar to other Homo specimens, the overwhelming similarity to the other australopithecines cannot be ignored. Berger et al. obviously didn’t ignore them and classified sediba as an australopithecine. I would agree. Regardless of the bootstrap BDIST calculations, sediba should not be considered a human.
Biblical Implications of sediba being a Human
Wood would have us believe that sediba represents post-Flood diversification and the sediba traits are just variation within the human line. This would mean that sediba is a descendant of Adam and Eve as well as Noah. Further, since sediba was found in South Africa, it is a descendant of Noah that made it quite some distance from anyone’s suggested location for the Tower of Babel. Given the numerous and strong similarity to africanus and the other australopithecines, one begins to wonder what traits pre-Flood humans would have had. If rudolfensis and habilis led the post-Babel dispersal, were pre-Flood humans like sahelanthropus?
One of the common definitions of species is a group of organisms capable of interbreeding and producing fertile offspring. According to the Bible, it is clear that all humans are descended from Adam and Eve and should therefore be considered a single reproducing population. Wood’s suggestion that there are at least eight species that could be considered human is clearly at odds with a single human species.
Baraminology Issues
Baraminology suffers from similar problems and limitations that cladistics does. These are intrinsic to the method and cannot be avoided. Statistical baraminology may have advantages over traditional cladistics because of the ability to test multiple characters simultaneously, as well as three-dimensional representations as opposed to the typical branching diagram. Nonetheless, there is no objective, independent validation that can be done to demonstrate that false negatives and false positives do not occur. In other words, there can be strong similarities without baraminological relatedness as well as significant differences. Bootstrapping helps, but one is still required to sample from the same dataset. Wood acknowledges this with a citation to one of his previous papers reviewing several different studies.
Wood acknowledges that statistical baraminology works best with different types of characters, yet the conclusions of this paper are only based on craniodental data. While this obviously is all we have to go on for specimens like rudolfensis, this means the conclusions need to be drawn extremely tentatively until further validation can be made. This makes the strong conclusion that there should be eight species that can be considered human quite a stretch.
Both baraminology and cladistics treat all characters with equal weights. However, certain traits will be affected by others. For example, a robust jaw will require a larger jaw muscle (DeWitt 2005). The larger jaw muscle will impact the size of the zygomatic arch as well as facial dimensions. So what might seem like several independent characters may not really be independent. In such cases then, weighting of characters is in fact occurring without the knowledge of the investigator. A further complication is that some traits should carry more weight. For example, with Australopithecus sediba and Ardipithecus ramidus researchers have focused on aspects of the pelvis and the femur angle to support their conclusion that these creatures walked upright. However, for arboreal individuals who will walk on tree branches like a tight rope, it would make sense to have these features in common with bipedal individuals. Other traits such as a rear-angled foramen magnum or an opposable big toe are consistent with knuckle walking. When it comes to determining means of locomotion, a rear-angled foramen magnum is more significant than the femur angle and thus carries more weight. These and other factors are ignored in statistical baraminology when all traits are treated equally.
Other problems highlight the limitations of statistical baraminology. Apes such as the gorilla have striking sexual dimorphism. The extent of dimorphism in fossil hominids is not clear, especially for fossils for which we only have one specimen. There are no error bars provided for craniodental measurement data such as those of Berger et al. Thus the true range of possible variation is unknown. Instead of a representative cloud in the 3D figures, specimens are reduced to a discreet value. This is further complicated by the descriptions of certain traits as: strong, weak, slight, marked, low, high, etc. (Berger et al. 2010). Such descriptions are useful for comparisons and can be informative. However, assigning a value to them is difficult, and variation is lost. What is the boundary between weak and strong? For any cases that are close it could be arbitrary and yet have implications for statistical baraminology.
Other issues
Kenyanthropus was excluded from the analysis. Wood justifies the exclusion, but inclusion could have a significant impact on the conclusions of this study.
The original reconstruction of the 1470 Homo rudolfensis skull has been questioned by modeling of craniofacial development by Bromage et al. (2008). In particular the cranial capacity should be reduced and the facial angle increased. These changes would bring 1470 more in line with australopithecines.
A large number of traits and a large number of individual fossils were excluded from the analysis. Wood justifies the exclusion and provides criteria for doing so. He also reports that two of the datasets he analyzed were uninformative. This does not facilitate placing much confidence in the datasets that were informative.
Wood emphasizes that DNA sequences from Neanderthals show that they are genetically distinct from modern humans. This becomes hard to reconcile when they are much more morphologically similar to modern humans than sediba. Fortunately, the draft sequence of the full Neandertal genome was reported two days after Wood’s paper appeared, and provided strong evidence of interbreeding between Neandertal's and modern humans (Green et al. 2010). Since this study appeared after Wood’s paper, he cannot be held accountable for it. Nonetheless, it should be included in any response.
Conclusion
Based on the discussion above, it would appear that the statistical baraminology results placing Homo habilis, Homo rudolfensis, and Australopithecus sediba within the human holobaramin is a false positive result. This is most clearly demonstrated for sediba and therefore raises questions about habilis and rudolfensis.
References
Balter, M. 2010. Candidate human ancestor from South Africa sparks praise and debate. Science 328, no. 5975:154–155.
Berger, L. R., D. J. de Ruiter, S. E. Churchill, P. Schmid, K. J. Carlson, P. H. G. M. Dirks, and J. M. Kibii. 2010. Australopithecus sediba: A new species of homo-like australopith from South Africa. Science 328, no. 5975:195–204.
Bromage, T. G., J. M. McMahon, J. F. Thackeray, O. Kullmer, R. Hogg, A. L. Rosenberger, F. Schrenk, and D. H. Enlow. 2008. Craniofacial architectural constraints and their importance for reconstructing the early Homo skull KNM-ER 1470. Journal of Clinical Pediatric Dentistry 33, no. 1:43–54.
Cuozzo, J. W. 1998. Buried alive. Green Forest, Arkansas: Master Books.
DeWitt, D. A. 2005. Did a jaw muscle protein mutation lead to increased cranial capacity in man? TJ (formerly Creation Ex Nihilo Technical Journal) 19, no. 1:88–96.
EverythingBio—an all encompassing biology resource. Retrieved from, http://www.everythingbio.com/glos/definition.php?word=cladistics.
Foley, J. 2008. Comparison of all skulls. Retrieved June 21, 2010, from Talk Origins online: http://www.talkorigins.org/faqs/homs/compare.html.
Green, R. E. et al. 2010. A draft sequence of the Neandertal genome. Science 328, no. 5979:710–722.
Habermehl, A. 2010. Those Enigmatic Neanderthals: What Are They Saying? Are We Listening? Answers Research Journal 3:1–21.
Line, P. 2010. Australopithecus sediba—no human ancestor. Retrieved from, http://creation.com/sediba-not-human-ancestor.
Lubenow, M. L. 2004. Bones of contention: A creationist assessment of human fossils, 2nd ed. Grand Rapids, Michigan: Baker.
Matzke, N. 2010. Retrieved on June 30, 2010 from, http://pandasthumb.org/archives/2010/05/creationist-vs.html.
Thomas, B. 2010. A new evolutionary link? Australopithecus sediba has all the wrong signs. Retrieved from, http://www.icr.org/article/new-evolutionary-link-australopithecus/.
Thomas, B. 2010. Australopithecus sediba: Another human ancestor? Retrieved from, http://www.icr.org/article/australopithecus-sediba-another-human/.
Williams, A. 2004. Baraminology, biology, and the Bible. TJ (formerly Creation Ex Nihilo Technical Journal) 18, no. 2:53–54.
Wood, T. C. 2010. Baraminological Analysis Places Homo habilis, Homo rudolfensis, and Australopithecus sediba in the Human Holobaramin. Answers Research Journal 3:71–90.
Wood, T. C. and M. J. Murray. 2003. Understanding the pattern of life. Nashville, Tennessee: Broadman & Holman.